Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 07, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 131:
Neutrons penetrate matter easily because they:
A. occupy no more than one-tenth of the volume of the electrons.
B. are electrically neutral.
C. occupy no more than one-tenths of the volume of the protons.
D. have smaller mass than protons.
-
Question 132:
The frequency of second's pendulum is:
A. 2 Hertz
B. 0.5 Hertz
C. 1 Hertz
D. 0.25 Hertz
-
Question 133:
If tension in the string remains constant but the diameter of the sitting is doubled, what will be the effect on speed of transverse waves in the string?
A. It remains constant.
B. It becomes double.
C. It becomes half.
D. It decreases four times.
-
Question 134:
Pressure exerted on a surface with some force is:
A. directly proportional to the surface area.
B. inversely proportional to the surface area.
C. directly proportional to the force.
D. None of them.
-
Question 135:
The equation for Ampere law is:
A. µoI = Br
B. µoI = Br2
C. µoI = B2r
D. µoI = B2r2
-
Question 136:
A rate of change of angular momentum of a body is equivalent to:
A. impulsive force.
B. the applied torque.
C. applied force.
D. moment of inertia.
-
Question 137:
Which of the following is equivalent to one Pascal of gas pressure?
A. 1 Kg.m2/s2
B. 1 Kg.m3/s2
C. 1 Kg/m.s2
D. 1 Kg.m/s
-
Question 138:
When a radio station is broadcasting a musical program, the antenna of its transmitter:
A. radiates AF electromagnetic waves.
B. radiates longitudinal waves.
C. radiates RF electromagnetic waves.
D. radiates AF longitudinal waves.
-
Question 139:
A copper rod has a resistance of 40 at 00C and a resistance of 56 at 1000C. What is the temperature coefficient of resistance of copper?
A. 2 x 10-4 K-1
B. 4 x 10-3 K-1
C. 4 x 10-4 K-1
D. 8 x 10-4 K-1
-
Question 140:
A student conducts a chemical analysis of the components of a popular soft drink. The beverage label shows that the drink contains carbonated water, phosphoric acid, caffeine, and caramel color, but does not indicate the concentrations of these chemicals.
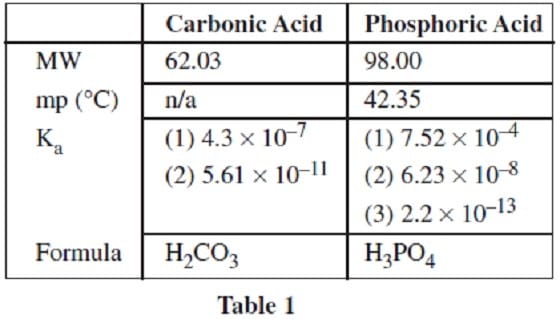
Dissolved carbon dioxide will react reversibly with water to form carbonic acid. In an attempt to analyze the beverage composition, the student conducts the following experiments on a one liter sample of the beverage.
Experiment 1
The sample is placed in a sealed beaker cooled to 10?C and a vacuum is created in the space above the beverage. The gas pumped from this space is passed through a solution of BaCl2, producing a white precipitate. The process
continues until no more precipitate forms. The precipitate is dried and found to have a mass of 9.5 grams.
Experiment 2
The remaining solution left in the sealed beaker is then titrated with 0.01 M NaOH to give the titration curve shown in Figure 1.

Figure 1
The student uses the data from Experiment 1 and Experiment 2 to calculate the initial pH of the beverage. If a significant quantity of precipitate was lost in the drying process of Experiment 1, the calculated pH:
A. would be less than the actual pH.
B. would be greater than the actual pH.
C. would be the same as the actual pH.
D. would differ from the actual pH in a random manner.
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.