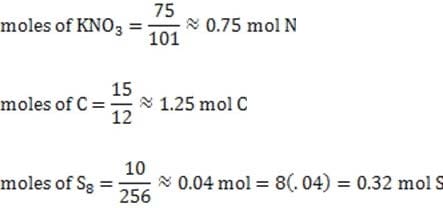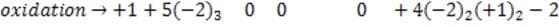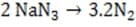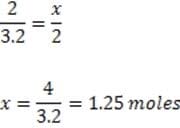Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 201:
Fireworks have been used for centuries in celebrations around the world. One of the primary components of these devices, black powder, was developed by the Chinese over a thousand years ago and is still used today as a propellant and explosive. Black powder is composed of potassium nitrate (KNO3), charcoal (primarily C) and sulfur (S8) in a 75:15:10 ratio by weight. It is very stable if kept dry but can easily be ignited by a spark or burning fuse to undergo the following reaction:
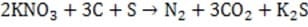
Reaction 1 The basic firework is shown in Figure 1. Fireworks rely on a particular kind of combustion in which oxygen is supplied by oxidizing agents included in the pyrotechnic mixture. When ignited, the solid propellant begins to liquefy and vaporize allowing the fuel and oxidizing agents to interact more intimately leading to rapid expansion of gases. Delay fuses time the ignition of the other compartments to occur when the shell is high above ground.
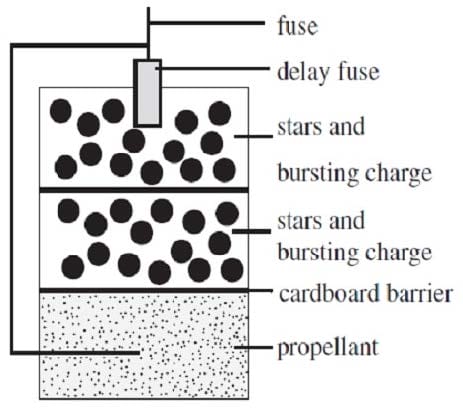
Figure 1
The light generating units of the firework are called stars and are dispersed and ignited by the bursting charge in each compartment. The intense colors of modern fireworks are generated by molecular emitters. For example, barium chloride emits green light (510?30 nm) and strontium chloride emits vibrant red light (605?50 nm). Many of the molecular emitters are unstable at room temperature and so cannot be placed directly into the firework. Instead, they are synthesized in the flame of the pyrotechnic reaction and exist for a short time before decomposing. The flame temperature must be carefully adjusted so that these emitters do not decompose too rapidly.
The molar ratio of N to C to S in black powder is approximately:
A. 75:125:35
B. 85:10:210
C. 75:15:10
D. 100:20:7
-
Question 202:
Fireworks have been used for centuries in celebrations around the world. One of the primary components of these devices, black powder, was developed by the Chinese over a thousand years ago and is still used today as a propellant and explosive. Black powder is composed of potassium nitrate (KNO3), charcoal (primarily C) and sulfur (S8) in a 75:15:10 ratio by weight. It is very stable if kept dry but can easily be ignited by a spark or burning fuse to undergo the following reaction:
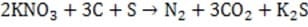
Reaction 1 The basic firework is shown in Figure 1. Fireworks rely on a particular kind of combustion in which oxygen is supplied by oxidizing agents included in the pyrotechnic mixture. When ignited, the solid propellant begins to liquefy and vaporize allowing the fuel and oxidizing agents to interact more intimately leading to rapid expansion of gases. Delay fuses time the ignition of the other compartments to occur when the shell is high above ground.

Figure 1
The light generating units of the firework are called stars and are dispersed and ignited by the bursting charge in each compartment. The intense colors of modern fireworks are generated by molecular emitters. For example, barium chloride emits green light (510?30 nm) and strontium chloride emits vibrant red light (605?50 nm). Many of the molecular emitters are unstable at room temperature and so cannot be placed directly into the firework. Instead, they are synthesized in the flame of the pyrotechnic reaction and exist for a short time before decomposing. The flame temperature must be carefully adjusted so that these emitters do not decompose too rapidly.
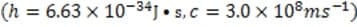
Which of the following chemicals is oxidized in the pyrotechnic reaction of gunpowder (Reaction 1)?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 203:
Fireworks have been used for centuries in celebrations around the world. One of the primary components of these devices, black powder, was developed by the Chinese over a thousand years ago and is still used today as a propellant and explosive. Black powder is composed of potassium nitrate (KNO3), charcoal (primarily C) and sulfur (S8) in a 75:15:10 ratio by weight. It is very stable if kept dry but can easily be ignited by a spark or burning fuse to undergo the following reaction:
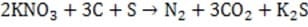
Reaction 1 The basic firework is shown in Figure 1. Fireworks rely on a particular kind of combustion in which oxygen is supplied by oxidizing agents included in the pyrotechnic mixture. When ignited, the solid propellant begins to liquefy and vaporize allowing the fuel and oxidizing agents to interact more intimately leading to rapid expansion of gases. Delay fuses time the ignition of the other compartments to occur when the shell is high above ground.

Figure 1
The light generating units of the firework are called stars and are dispersed and ignited by the bursting charge in each compartment. The intense colors of modern fireworks are generated by molecular emitters. For example, barium chloride emits green light (510?30 nm) and strontium chloride emits vibrant red light (605?50 nm). Many of the molecular emitters are unstable at room temperature and so cannot be placed directly into the firework. Instead, they are synthesized in the flame of the pyrotechnic reaction and exist for a short time before decomposing. The flame temperature must be carefully adjusted so that these emitters do not decompose too rapidly.
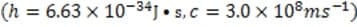
Which of the following bonds has the greatest ionic character?
A. Ba–Cl
B. Sr–Cl
C. N–N
D. O=CO
-
Question 204:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
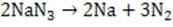
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
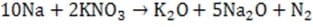
Reaction 2
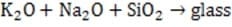
Reaction 3
All of the following are resonance structures of N3- EXCEPT:
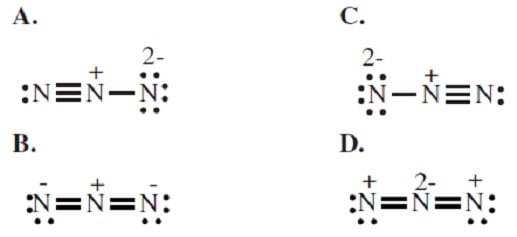
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 205:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
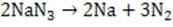
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
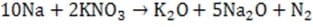
Reaction 2
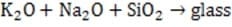
Reaction 3
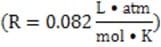
A researcher wishes to make the decomposition of sodium azide (Reaction 1) less favorable. Which of the following adjustments to the reaction would NOT drive it to the left?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 206:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
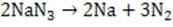
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
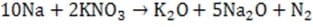
Reaction 2
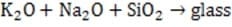
Reaction 3
Decomposition of which of the following transition metal complexes would produce the highest theoretical yield of carbon monoxide per gram of reactant?
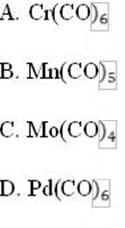
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 207:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
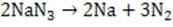
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
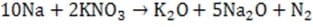
Reaction 2
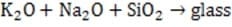
Reaction 3
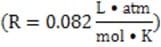
A sodium azide air bag inflates to a volume of 45 Liters at STP. According to the information contained in the passage, what is the mass of NaN3 (Mol. Wt. = 65) that is required to inflate the bag?
A. 76 grams
B. 81 grams
C. 87 grams
D. 130 grams
-
Question 208:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
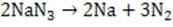
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
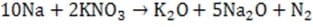
Reaction 2
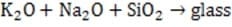
Reaction 3
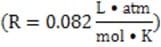
Potassium chlorate, KClO3(s), decomposes when heated, yet it is unsuitable as an airbag inflatant. All of the following characteristics of KClO3 make it a poor candidate for an air bag inflatant EXCEPT:
A. the decomposition requires a steady supply of energy due to its high activation energy and low exothermicity.
B. the oxygen gas formed upon decomposition creates a combustion hazard during an automobile accident.
C. the potassium chloride formed upon decomposition is a dense solid.
D. the oxygen gas formed upon decomposition leads to rapid expansion of the reaction mixture.
-
Question 209:
The automobile airbag was designed to inflate upon impact and decrease the risk of injury to drivers and passengers. Among the challenges to its development was the need to find a reliable inflation mechanism that was sufficiently rapid, controllable, and nontoxic. Prototypes employing compressed gases failed to meet these criteria. Researchers thus turned their attention to chemical alternatives.
The ideal inflatant requires a chemical reaction in which the reactants are stable and relatively dense in the condensed phase while the products are mostly or completely gaseous at ambient temperature and pressure. Additionally, the ideal chemical reaction would require a low activation energy and have a high kinetic rate constant, without the large exothermicity characteristic of most such reactions. Traditional explosives such as nitroglycerin, C3H5N3O9(l), were rejected almost immediately because of the extremely exothermic nature of their conversion. Benign solids such as calcium carbonate, CaCO3 , were similarly rejected, because of their large activation requirements. The desired attributes were finally found in sodium azide, NaN3, a stable, dense, ionic solid which rapidly decomposes into elemental sodium and nitrogen gas when ignited by an electrical impulse.
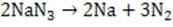
Reaction 1
The gas generating mixture includes excess KNO3 which reacts with the sodium metal from Reaction 1 to produce additional N2 and potassium and sodium oxides (Reactions 2 and 3). These oxides react with SiO2 to produce a non-toxic and stable alkaline silica (glass).
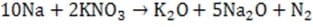
Reaction 2
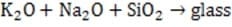
Reaction 3
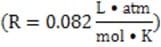
In order for the decomposition reaction to spread throughout the sodium azide after ignition, the H and G for Reaction 1 must be, respectively:
A. positive, negative
B. positive, positive
C. negative, negative
D. negative, positive
-
Question 210:
Historically, two different methods have been used to estimate the fluid pressure in capillary beds.
Method 1 A glass pipette is inserted into the capillary. The level of blood rising in the pipette is measured and used to calculate the pressure. Alternatively, an inert fluid of density can be placed in the pipette and its height h can be measured. The pressure in the capillary is given by gh, where g is the acceleration due to gravity.
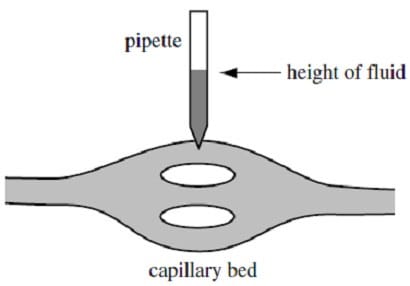
Figure 1 Method 2
The pressure can be measured indirectly in the following way. A section of gut tissue is removed from a specimen and placed on a beam balance. Blood is circulated through the tissue by a pump. The arterial pressure is then decreased. This leads to a decrease in the capillary hydrostatic pressure in the gut capillaries. The constant osmotic pressure of plasma proteins in the capillary causes absorption of fluid from the gut section which will decrease its weight. To prevent a change in the weight of the gut section, the venous pressure is increased. This tends to increase the capillary pressure, reducing the flow of fluid from the gut tissue into the capillaries. The capillary pressure is thus held constant (and the balance kept level) as the arterial pressure is decreased and the venous pressure increased. The arterial and venous pressures meet at the capillary pressure being measured.
( = MRT, where is the osmotic pressure, M the molarity of the solutes, R the universal gas constant, and T the temperature in Kelvin.)
Figure 2
Assume that the beam balance of Method 2 is initially level. If the arterial pressure is decreased to a lower level while everything else is held constant, which graph best represents the change in the mass of the gut following the decrease in arterial pressure?
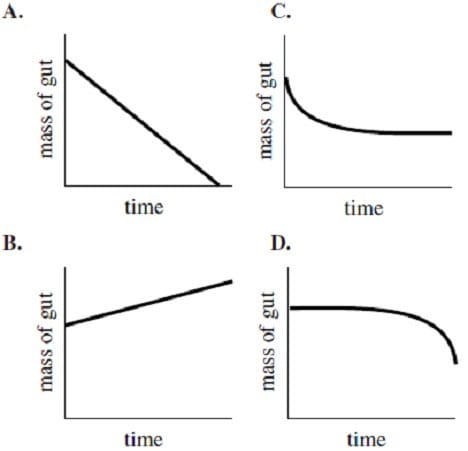
A. A
B. B
C. C
D. D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.