Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 221:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
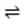
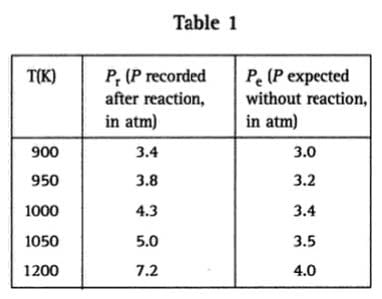
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1 How are the values of Pe calculated?
A. (T/273)(1 atm)
B. (T/298)(1 atm)
C. [(T ?273)/273](1 atm)
D. [(T ?298)/298](1 atm)
-
Question 222:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
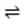
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1

If 0.6 g of elemental carbon are consumed during a trial, how many grams of CO are produced?
A. 0.8
B. 1.4
C. 1.6
D. 2.8
-
Question 223:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below: CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1
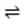

Which of the following is NOT necessarily true about the equilibrium reaction between CO2, C, and CO?
A. The standard entropy change is positive.
B. A decrease in pressure at constant temperature would shift the equilibrium to the right.
C. Addition of CO will shift the equilibrium to the left.
D. The standard Gibbs' free energy change is negative.
-
Question 224:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
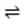
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1

Which of the following shows the correct Lewis structure of carbon monoxide?
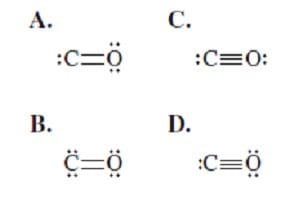
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 225:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
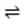
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1 When the system stabilized at 1,200 K, a sample of helium was injected into the furnace. What should happen to the amount of carbon dioxide in the system?

A. It should increase.
B. It should decrease.
C. It should be completely converted to carbon monoxide.
D. It should remain the same.
-
Question 226:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
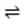
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1

What can be said about the value of S?of the reaction?
A. It is positive.
B. It is negative.
C. It is zero.
D. It cannot be determined from the information given.
-
Question 227:
A researcher investigated the equilibrium between CO2, C, and CO as a function of temperature. The equation is given below:
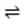
CO2(g) + C(s) 2 CO(g) Carbon dioxide, at 298 K and 1 atm, and an excess of powdered carbon were introduced into a furnace, which was then sealed so that pressure would increase as the temperature rose. The furnace was heated to, and held constant at, a predetermined temperature. The pressure within the furnace chamber was recorded after it had remained unchanged for one hour. The table below shows the pressures recorded for a series of temperatures together with the pressures expected if no reaction had taken place. Table 1

How many pi bonds are in the carbon dioxide molecule?
A. 0
B. 1
C. 2
D. 3
-
Question 228:
There are two opposing theories of light: the particle theory and the wave theory. According to the particle theory, light is composed of a stream of tiny particles that are subject to the same physical laws as other types of elementary particles.
One consequence of this is that light particles should travel in a straight line unless an external force acts on them. According to the wave theory, light is a wave that shares the characteristics of other waves. Among other things, this means
that light waves should interfere with each other under certain conditions.
In support of the wave theory of light, Thomas Young's double slit experiment proves that light does indeed exhibit interference. Figure 1 shows the essential features of the experiment. Parallel rays of monochromatic light pass through two
narrow slits and are projected onto a screen. Constructive interference occurs at certain points on the screen, producing bright areas of maximum light intensity. Between these maxima, destructive interference produces light intensity minima.
The positions of the maxima are given by the equation dsin = n, where d is the distance between the slits, is the angle shown in Figure 1, the integer n specifies the particular maxima, and is the wavelength of the incident light. (Note: sin tan
for small angles.).
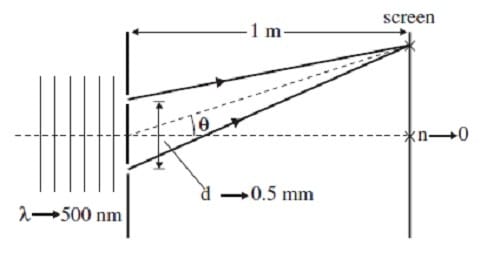
Figure 1
According to the modern theory of light, a beam of light may be described either as a stream of particles or as a wave, depending on the circumstances. Which of the following correctly states a connection between the two descriptions?
A. The number of light particles that pass by per second is proportional to the frequency of the light wave.
B. The mass of each particle of light is proportional to the intensity of the light wave.
C. The size of each particle of light is proportional to the wavelength of the light wave.
D. The energy of each particle of light is proportional to the frequency of the light wave.
-
Question 229:
There are two opposing theories of light: the particle theory and the wave theory. According to the particle theory, light is composed of a stream of tiny particles that are subject to the same physical laws as other types of elementary particles.
One consequence of this is that light particles should travel in a straight line unless an external force acts on them. According to the wave theory, light is a wave that shares the characteristics of other waves. Among other things, this means
that light waves should interfere with each other under certain conditions.
In support of the wave theory of light, Thomas Young's double slit experiment proves that light does indeed exhibit interference. Figure 1 shows the essential features of the experiment. Parallel rays of monochromatic light pass through two
narrow slits and are projected onto a screen. Constructive interference occurs at certain points on the screen, producing bright areas of maximum light intensity. Between these maxima, destructive interference produces light intensity minima.
The positions of the maxima are given by the equation dsin = n, where d is the distance between the slits, is the angle shown in Figure 1, the integer n specifies the particular maxima, and is the wavelength of the incident light. (Note: sin tan
for small angles.)
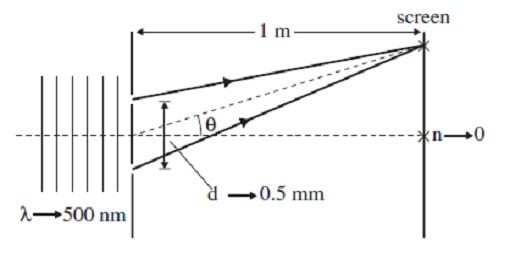
Figure 1
Light waves can be described in terms of frequency f and wavelength or in terms of wave number k and angular frequency . These quantities are related by the following equations:
k = 2/ and = 2f
Which equation below accurately describes the speed of the wave v in terms of k and ?
A. v = f
B. v = + k
C. v = /k
D. v = k
-
Question 230:
There are two opposing theories of light: the particle theory and the wave theory. According to the particle theory, light is composed of a stream of tiny particles that are subject to the same physical laws as other types of elementary particles.
One consequence of this is that light particles should travel in a straight line unless an external force acts on them. According to the wave theory, light is a wave that shares the characteristics of other waves. Among other things, this means
that light waves should interfere with each other under certain conditions.
In support of the wave theory of light, Thomas Young's double slit experiment proves that light does indeed exhibit interference. Figure 1 shows the essential features of the experiment. Parallel rays of monochromatic light pass through two
narrow slits and are projected onto a screen. Constructive interference occurs at certain points on the screen, producing bright areas of maximum light intensity. Between these maxima, destructive interference produces light intensity minima.
The positions of the maxima are given by the equation dsin = n, where d is the distance between the slits, is the angle shown in Figure 1, the integer n specifies the particular maxima, and is the wavelength of the incident light. (Note: sin tan
for small angles.)
Figure 1
Which of the following is sufficient information to determine the approximate speed of a ray of light in water?
A. The angle of incidence and the angle of refraction of the light ray as it enters water from air
B. The wavelength in water and the wavelength in air of the light ray as it enters water from air
C. The speed of light in a vacuum and the density of water
D. The speed of light in a vacuum and the index of refraction of water
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.