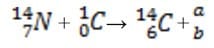Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 241:
If a spring is 64 cm long when it is unstretched and is 8% longer when a 0.5-kg mass hangs from it, how long will it be with a 0.4-kg mass suspended from it?
A. 66 cm
B. 68 cm
C. 70 cm
D. 74 cm
-
Question 242:
Which of the following will halve the magnitude of the electrostatic force of attraction between two charged particles?
A. Doubling the distance between the particles
B. Halving the charge on each particle
C. Halving the charge on only one of the particles
D. Placing a positively charged particle midway between the particles
-
Question 243:
Arsenic is widely distributed in sulfide ores of many metals and is obtained as a byproduct of copper smelting. The element, as well as many compounds of arsenic -- for example arsine, AsH3 -- are extremely poisonous. Arsenic compounds, as might be expected, have found use in herbicides and pesticides, but have also been successful in some pharmacological agents. The first useful antisyphilitic agent, Salvarsan, or 3,3'-diamino-4,4'-dihydroxyarsenobenzene dihydrochloride, is an arsenic compound. The element sublimes at 600°C, forming tetrahedral molecules, As4. Arsenic is a metalloid, possessing properties characteristic of both metals and non-metals. Arsenic is a gray-colored, metalliclooking solid, but arsenic vapor is yellow in color, has a garlic-like odor, and is very poisonous. If the arsenic vapor is cooled rapidly, an unstable, yellow crystalline allotrope consisting of As4 molecules is produced. The Marsh test, based on the instability of arsine, is a very sensitive test for the presence of arsenic. This test is commonly employed in the detection of arsenic poisoning -- either before or after death. The apparatus for the Marsh test is shown in Figure 1.
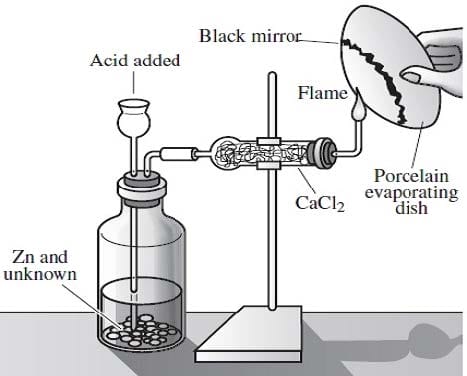
Figure 1
Typically, a sample, usually hair, is taken from a person suspected of being the victim of arsenic poisoning. This sample is then treated in such a way so as to produce arsenic oxide, As4O6. The oxide is then placed into the apparatus shown
in Figure 1 and reacted according to Reaction 1.
As4O6 + 12Zn(s) + 24H+(aq) AsH3(g) + 12Zn2 +(aq) + 6H2O
Reaction 1
When the evolved arsine is ignited it decomposes into its elements. The arsenic vapor is rapidly cooled when it encounters the porcelain evaporating dish and deposits a black mirror of arsenic on the bottom, indicating the presence of arsenic
in the original sample.
A common ore of arsenic is called orpiment, As2S3. What is the oxidation state of arsenic in orpiment?
A. ?
B. 0
C. +3
D. +6
-
Question 244:
What is the shape of a molecule of NH3?
A. Trigonal planar
B. Pyramidal
C. Tetrahedral
D. Trigonal bipyramidal
-
Question 245:
Arsenic is widely distributed in sulfide ores of many metals and is obtained as a byproduct of copper smelting. The element, as well as many compounds of arsenic -- for example arsine, AsH3 -- are extremely poisonous. Arsenic compounds, as might be expected, have found use in herbicides and pesticides, but have also been successful in some pharmacological agents. The first useful antisyphilitic agent, Salvarsan, or 3,3'-diamino-4,4'-dihydroxyarsenobenzene dihydrochloride, is an arsenic compound. The element sublimes at 600°C, forming tetrahedral molecules, As4. Arsenic is a metalloid, possessing properties characteristic of both metals and non-metals. Arsenic is a gray-colored, metalliclooking solid, but arsenic vapor is yellow in color, has a garlic-like odor, and is very poisonous. If the arsenic vapor is cooled rapidly, an unstable, yellow crystalline allotrope consisting of As4 molecules is produced. The Marsh test, based on the instability of arsine, is a very sensitive test for the presence of arsenic. This test is commonly employed in the detection of arsenic poisoning -- either before or after death. The apparatus for the Marsh test is shown in Figure 1.
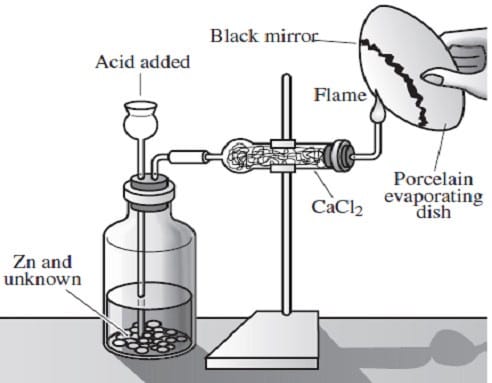
Figure 1
Typically, a sample, usually hair, is taken from a person suspected of being the victim of arsenic poisoning. This sample is then treated in such a way so as to produce arsenic oxide, As4O6. The oxide is then placed into the apparatus shown in Figure 1 and reacted according to Reaction 1. As4O6 + 12Zn(s) + 24H+(aq) AsH3(g) + 12Zn2 +(aq) + 6H2O Reaction 1 When the evolved arsine is ignited it decomposes into its elements. The arsenic vapor is rapidly cooled when it encounters the porcelain evaporating dish and deposits a black mirror of arsenic on the bottom, indicating the presence of arsenic in the original sample.
If equal masses of gray arsenic and yellow arsenic are allowed to completely react with oxygen at 298 K and constant pressure to form As4O6, which would produce more heat and why?
A. The yellow, because it is less stable than the gray.
B. The gray, because it is more stable than the yellow.
C. Both would produce the same amount of heat because they form the same product.
D. Both would produce the same amount of heat because they are the same element.
-
Question 246:
Arsenic is widely distributed in sulfide ores of many metals and is obtained as a byproduct of copper smelting. The element, as well as many compounds of arsenic -- for example arsine, AsH3 -- are extremely poisonous. Arsenic compounds, as might be expected, have found use in herbicides and pesticides, but have also been successful in some pharmacological agents. The first useful antisyphilitic agent, Salvarsan, or 3,3'-diamino-4,4'-dihydroxyarsenobenzene dihydrochloride, is an arsenic compound. The element sublimes at 600°C, forming tetrahedral molecules, As4. Arsenic is a metalloid, possessing properties characteristic of both metals and non-metals. Arsenic is a gray-colored, metalliclooking solid, but arsenic vapor is yellow in color, has a garlic-like odor, and is very poisonous. If the arsenic vapor is cooled rapidly, an unstable, yellow crystalline allotrope consisting of As4 molecules is produced. The Marsh test, based on the instability of arsine, is a very sensitive test for the presence of arsenic. This test is commonly employed in the detection of arsenic poisoning -- either before or after death. The apparatus for the Marsh test is shown in Figure 1.
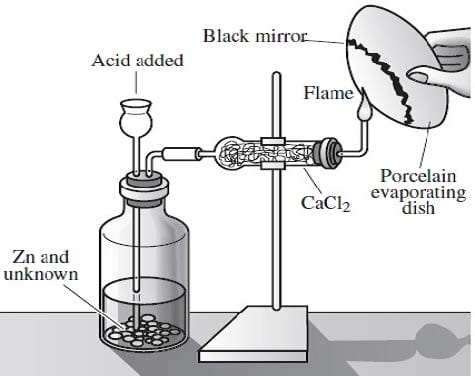
Figure 1
Typically, a sample, usually hair, is taken from a person suspected of being the victim of arsenic poisoning. This sample is then treated in such a way so as to produce arsenic oxide, As4O6. The oxide is then placed into the apparatus shown
in Figure 1 and reacted according to Reaction 1.
As4O6 + 12Zn(s) + 24H+(aq) AsH3(g) + 12Zn2 +(aq) + 6H2O
Reaction 1
When the evolved arsine is ignited it decomposes into its elements. The arsenic vapor is rapidly cooled when it encounters the porcelain evaporating dish and deposits a black mirror of arsenic on the bottom, indicating the presence of arsenic
in the original sample.
The Marsh test takes advantage of the fact that arsine is not very soluble in water. Since arsenic is below nitrogen on the periodic table, it would be expected that arsine, like ammonia, would be very soluble in water. What is the most likely
reason for this difference in solubility?
A. Arsine has a higher molecular weight than ammonia does.
B. Arsine has a smaller dipole moment than ammonia does.
C. Arsine is less basic than ammonia is.
D. Arsine is less stable than ammonia is.
-
Question 247:
Arsenic is widely distributed in sulfide ores of many metals and is obtained as a byproduct of copper smelting. The element, as well as many compounds of arsenic -- for example arsine, AsH3 -- are extremely poisonous. Arsenic compounds, as might be expected, have found use in herbicides and pesticides, but have also been successful in some pharmacological agents. The first useful antisyphilitic agent, Salvarsan, or 3,3'-diamino-4,4'-dihydroxyarsenobenzene dihydrochloride, is an arsenic compound. The element sublimes at 600°C, forming tetrahedral molecules, As4. Arsenic is a metalloid, possessing properties characteristic of both metals and non-metals. Arsenic is a gray-colored, metalliclooking solid, but arsenic vapor is yellow in color, has a garlic-like odor, and is very poisonous. If the arsenic vapor is cooled rapidly, an unstable, yellow crystalline allotrope consisting of As4 molecules is produced. The Marsh test, based on the instability of arsine, is a very sensitive test for the presence of arsenic. This test is commonly employed in the detection of arsenic poisoning--either before or after death. The apparatus for the Marsh test is shown in Figure 1.
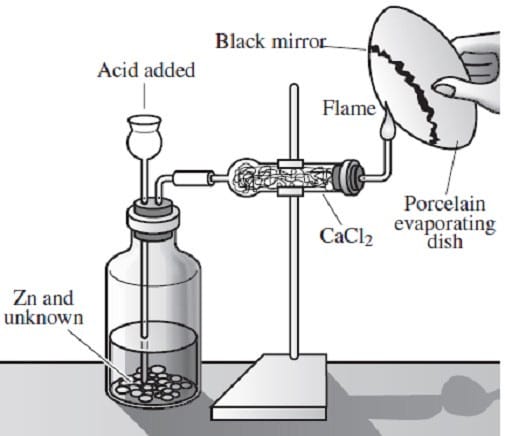
Figure 1
Typically, a sample, usually hair, is taken from a person suspected of being the victim of arsenic poisoning. This sample is then treated in such a way so as to produce arsenic oxide, As4O6. The oxide is then placed into the apparatus shown
in Figure 1 and reacted according to Reaction 1.
As4O6 + 12Zn(s) + 24H+(aq) AsH3(g) + 12Zn2 +(aq) + 6H2O
Reaction 1
When the evolved arsine is ignited it decomposes into its elements. The arsenic vapor is rapidly cooled when it encounters the porcelain evaporating dish and deposits a black mirror of arsenic on the bottom, indicating the presence of arsenic
in the original sample.
The phase diagram for arsenic is shown below. At what point does liquid arsenic exist?
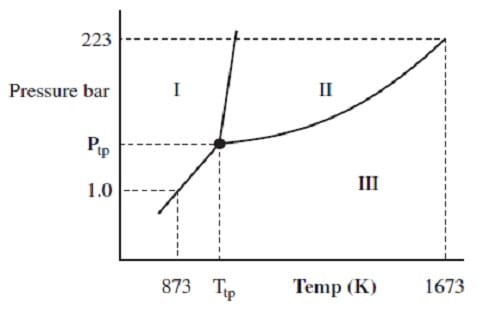
A. 1.0 bar and 874 K
B. 1.0 bar and 1673 K
C. 223 bar and 1672 K
D. 223 bar and 873 K
-
Question 248:
Arsenic is widely distributed in sulfide ores of many metals and is obtained as a byproduct of copper smelting. The element, as well as many compounds of arsenic -- for example arsine, AsH3 -- are extremely poisonous. Arsenic compounds, as might be expected, have found use in herbicides and pesticides, but have also been successful in some pharmacological agents. The first useful antisyphilitic agent, Salvarsan, or 3,3'-diamino-4,4'-dihydroxyarsenobenzene dihydrochloride, is an arsenic compound. The element sublimes at 600°C, forming tetrahedral molecules, As4. Arsenic is a metalloid, possessing properties characteristic of both metals and non-metals. Arsenic is a gray-colored, metalliclooking solid, but arsenic vapor is yellow in color, has a garlic-like odor, and is very poisonous. If the arsenic vapor is cooled rapidly, an unstable, yellow crystalline allotrope consisting of As4 molecules is produced. The Marsh test, based on the instability of arsine, is a very sensitive test for the presence of arsenic. This test is commonly employed in the detection of arsenic poisoning--either before or after death. The apparatus for the Marsh test is shown in Figure 1.
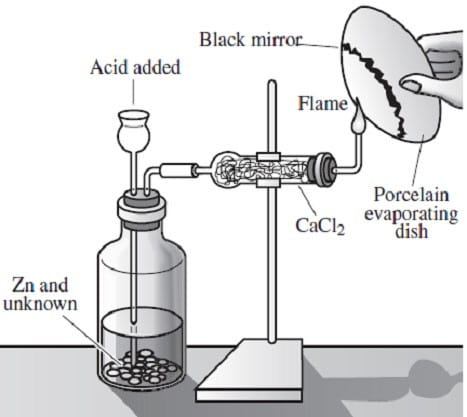
Figure 1 Typically, a sample, usually hair, is taken from a person suspected of being the victim of arsenic poisoning. This sample is then treated in such a way so as to produce arsenic oxide, As4O6. The oxide is then placed into the apparatus shown in Figure 1 and reacted according to Reaction 1. As4O6 + 12Zn(s) + 24H+(aq) AsH3(g) + 12Zn2 +(aq) + 6H2O Reaction 1
When the evolved arsine is ignited it decomposes into its elements. The arsenic vapor is rapidly cooled when it encounters the porcelain evaporating dish and deposits a black mirror of arsenic on the bottom, indicating the presence of arsenic in the original sample.
What is the most likely purpose of the calcium chloride in Figure 1?
A. To remove water from the evolved arsine gas
B. To remove HCl from the evolved arsine gas
C. To react with the zinc ion, making the reaction go to completion
D. To react with the evolved arsine gas
-
Question 249:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of 14C that was present in the organism when it died, the age of the fossil can be determined.
In generating 14C in the upper atmosphere, a 14C nucleus combines with a neutron to form a 14C nucleus and:
A. a proton.
B. an electron.
C. a 4He nucleus.
D. a neutron.
-
Question 250:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of 14C that was present in the organism when it died, the age of the fossil can be determined.
After a 14C nucleus decays, the electron that is emitted enters lead and is stopped. What percentage of its kinetic energy does the electron transfer to lead?
A. 25%
B. 33%
C. 50%
D. 100%
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.