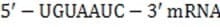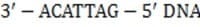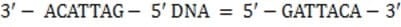Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 531:
Synthetic dyes constitute a commercially significant area of organic chemistry. The color producing properties of these compounds are the result of highly delocalized electron systems giving rise to electronic transitions whose absorptions occur in the visible region. Most commercially useful dyes can be classified as one of three types -- anthraquinones, azo dyes, or triarylmethyl salts. Examples of each type are illustrated in Figure 1.
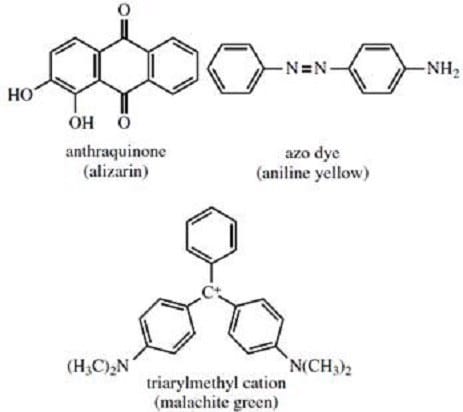
Figure 1
In order for a dye to be useful in the fabric industry, it must have sufficient affinity for the polymeric fibers of which the material is composed; the dye must not only impart a color to the fabric, but must also do so in a relatively permanent manner (color fastness). Proper design of synthetic polymers requires the placement of acidic or basic side chains along the polymer backbone such that binding sites are available for dying. Similarly, dyes must be produced not only with the appropriate color-producing structure, but also with an affinity for the fabric in question. The structural units of several common synthetic fibers are shown in Figure 2.
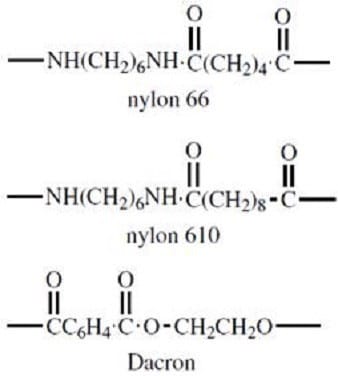
Figure 2
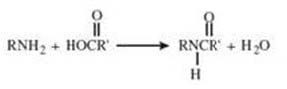
Nylon, Dacron, and many other synthetic fibers are produced via condensation reactions. Which of the following would be the best starting materials for the production of nylon 66?
A. cis-2-butenoic acid and 1,6-hexanediamine
B. butanedioic acid and 1,6-hexanediamine
C. n-hexanoic acid and 2,5-hexanediamine
D. hexanedioic acid and 1,6-hexanediamine
-
Question 532:
Synthetic dyes constitute a commercially significant area of organic chemistry. The color producing properties of these compounds are the result of highly delocalized electron systems giving rise to electronic transitions whose absorptions occur in the visible region. Most commercially useful dyes can be classified as one of three types -- anthraquinones, azo dyes, or triarylmethyl salts. Examples of each type are illustrated in Figure 1.

Figure 1
In order for a dye to be useful in the fabric industry, it must have sufficient affinity for the polymeric fibers of which the material is composed; the dye must not only impart a color to the fabric, but must also do so in a relatively permanent manner (color fastness). Proper design of synthetic polymers requires the placement of acidic or basic side chains along the polymer backbone such that binding sites are available for dying. Similarly, dyes must be produced not only with the appropriate color-producing structure, but also with an affinity for the fabric in question. The structural units of several common synthetic fibers are shown in Figure 2.

Figure 2
Dacron belongs to which of the following general classifications?
A. Polyamide
B. Polyester
C. Polyurethane
D. Polypeptide
-
Question 533:
Synthetic dyes constitute a commercially significant area of organic chemistry. The color producing properties of these compounds are the result of highly delocalized electron systems giving rise to electronic transitions whose absorptions occur in the visible region. Most commercially useful dyes can be classified as one of three types -- anthraquinones, azo dyes, or triarylmethyl salts. Examples of each type are illustrated in Figure 1.

Figure 1
In order for a dye to be useful in the fabric industry, it must have sufficient affinity for the polymeric fibers of which the material is composed; the dye must not only impart a color to the fabric, but must also do so in a relatively permanent manner (color fastness). Proper design of synthetic polymers requires the placement of acidic or basic side chains along the polymer backbone such that binding sites are available for dying. Similarly, dyes must be produced not only with the appropriate color-producing structure, but also with an affinity for the fabric in question. The structural units of several common synthetic fibers are shown in Figure 2.

Figure 2
Cotton is a natural fiber composed of cellulose, a polymer of glucose. Which of the compounds shown in Figure 1 would adhere to a cotton fiber via hydrogen bonding?
A. Alizarin only
B. Malachite green only
C. Alizarin and aniline yellow only
D. Malachite green, alizarin, and aniline yellow
-
Question 534:
Nitric oxide, NO, has recently been found to have widespread physiological effects, acting as a major regulator in the nervous, cardiovascular, and immune systems. The production of NO in the body is regulated by specific NOS enzymes which exist in at least three different isoforms -- bNOS, eNOS, and macNOS. Each of these isoforms differ in location and function and serve to mediate different physiological responses to NO. Some physiological roles of NO have been demonstrated as follows:
I. In the central nervous system, NO production is regulated by bNOS. Calcium ion concentrations of 200- 400 nM in the central nervous system activate bNOS to catalyze the formation of NO. NO exerts definite effects on brain function although its specific roles are not well established. bNOS inhibitors have been found to block the release of neurotransmitter from presynaptic neurons. Excess levels of NO are also thought to contribute to neurodegenerative disorders such as Alzheimer's disease.
II. In the blood vessels, NO is produced by eNOS which is activated by Ca2+ concentrations of 200-400 nM. NO acts as the major endogenous vasodilator in blood vessels. It diffuses into smooth muscle cells and leads to muscle relaxation by stimulating cGMP formation through activation of guanylyl cyclase. In addition, NO regulates the vascular system by inhibiting platelet aggregation and adhesion.
III. The role of NO in the immune system is regulated by macNOS through a pathway that is not Ca2+ dependent. Rather, exposure to cytokines, including interleukin-1 and interferon- , leads to synthesis of large amounts of NO by activation of macNOS in response to inflammatory stimuli. The NO produced plays a definitive role in the mediation of the activities of macrophages and neutrophils. NO also acts to inhibit the mechanism of viral replication.
According to the passage, NO may counter viral infection by all of the following mechanisms EXCEPT:
A. inducing white blood cells to engulf and destroy foreign agents.
B. inhibiting the synthesis of viral RNA.
C. denaturing viral protein coats in the interstitial fluid.
D. blocking viral release from infected cells.
-
Question 535:
Synthetic dyes constitute a commercially significant area of organic chemistry. The color producing properties of these compounds are the result of highly delocalized electron systems giving rise to electronic transitions whose absorptions occur in the visible region. Most commercially useful dyes can be classified as one of three types -- anthraquinones, azo dyes, or triarylmethyl salts. Examples of each type are illustrated in Figure 1.

Figure 1
In order for a dye to be useful in the fabric industry, it must have sufficient affinity for the polymeric fibers of which the material is composed; the dye must not only impart a color to the fabric, but must also do so in a relatively permanent manner (color fastness). Proper design of synthetic polymers requires the placement of acidic or basic side chains along the polymer backbone such that binding sites are available for dying. Similarly, dyes must be produced not only with the appropriate color-producing structure, but also with an affinity for the fabric in question. The structural units of several common synthetic fibers are shown in Figure 2.

Figure 2
Certain natural protein fibers such as silk or wool can be treated with aqueous base, then with solutions containing cationic dyes such as malachite green to produce color fast yarns. The most likely explanation for the affinity of malachite green for silk or wool via this process is that:
A. many of the R groups on the amino acids of which these fibers are composed contain COOH groups.
B. very few of the R groups on the amino acids of which these fibers are composed contain OH groups.
C. the aqueous base hydrolyzes some of the peptide linkages in these fibers.
D. the aqueous base neutralizes the cationic dye.
-
Question 536:
Nitric oxide, NO, has recently been found to have widespread physiological effects, acting as a major regulator in the nervous, cardiovascular, and immune systems. The production of NO in the body is regulated by specific NOS enzymes which exist in at least three different isoforms -- bNOS, eNOS, and macNOS. Each of these isoforms differ in location and function and serve to mediate different physiological responses to NO. Some physiological roles of NO have been demonstrated as follows:
I. In the central nervous system, NO production is regulated by bNOS. Calcium ion concentrations of 200- 400 nM in the central nervous system activate bNOS to catalyze the formation of NO. NO exerts definite effects on brain function although its specific roles are not well established. bNOS inhibitors have been found to block the release of neurotransmitter from presynaptic neurons. Excess levels of NO are also thought to contribute to neurodegenerative disorders such as Alzheimer's disease.
II. In the blood vessels, NO is produced by eNOS which is activated by Ca2+ concentrations of 200-400 nM. NO acts as the major endogenous vasodilator in blood vessels. It diffuses into smooth muscle cells and leads to muscle relaxation by stimulating cGMP formation through activation of guanylyl cyclase. In addition, NO regulates the vascular system by inhibiting platelet aggregation and adhesion.
III. The role of NO in the immune system is regulated by macNOS through a pathway that is not Ca2+ dependent. Rather, exposure to cytokines, including interleukin-1 and interferon- , leads to synthesis of large amounts of NO by activation of macNOS in response to inflammatory stimuli. The NO produced plays a definitive role in the mediation of the activities of macrophages and neutrophils. NO also acts to inhibit the mechanism of viral replication.
A patient has accidentally ingested a toxin which acts as an eNOS inhibitor. According to the passage, the effects of this toxin would most likely include:
A.
B. decreased blood pressure.
C. increased immune response.
D. decreased urine production.
E. increased blood pressure.
-
Question 537:
Nitric oxide, NO, has recently been found to have widespread physiological effects, acting as a major regulator in the nervous, cardiovascular, and immune systems. The production of NO in the body is regulated by specific NOS enzymes which exist in at least three different isoforms -- bNOS, eNOS, and macNOS. Each of these isoforms differ in location and function and serve to mediate different physiological responses to NO. Some physiological roles of NO have been demonstrated as follows:
I. In the central nervous system, NO production is regulated by bNOS. Calcium ion concentrations of 200- 400 nM in the central nervous system activate bNOS to catalyze the formation of NO. NO exerts definite effects on brain function although its specific roles are not well established. bNOS inhibitors have been found to block the release of neurotransmitter from presynaptic neurons. Excess levels of NO are also thought to contribute to neurodegenerative disorders such as Alzheimer's disease.
II. In the blood vessels, NO is produced by eNOS which is activated by Ca2+ concentrations of 200-400 nM. NO acts as the major endogenous vasodilator in blood vessels. It diffuses into smooth muscle cells and leads to muscle relaxation by stimulating cGMP formation through activation of guanylyl cyclase. In addition, NO regulates the vascular system by inhibiting platelet aggregation and adhesion.
III. The role of NO in the immune system is regulated by macNOS through a pathway that is not Ca2+ dependent. Rather, exposure to cytokines, including interleukin-1 and interferon- , leads to synthesis of large amounts of NO by activation of macNOS in response to inflammatory stimuli. The NO produced plays a definitive role in the mediation of the activities of macrophages and neutrophils. NO also acts to inhibit the mechanism of viral replication.
A patient with hypertension (high blood pressure) is treated with nitroglycerin, which spontaneously breaks down to give NO. This treatment will be:
A. effective because NO will activate eNOS.
B. effective because NO will lead to vasodilation.
C. ineffective because eNOS will not be activated.
D. ineffective because NO will build up, leading to neurotoxicity.
-
Question 538:
Nitric oxide, NO, has recently been found to have widespread physiological effects, acting as a major regulator in the nervous, cardiovascular, and immune systems. The production of NO in the body is regulated by specific NOS enzymes which exist in at least three different isoforms -- bNOS, eNOS, and macNOS. Each of these isoforms differ in location and function and serve to mediate different physiological responses to NO. Some physiological roles of NO have been demonstrated as follows:
I. In the central nervous system, NO production is regulated by bNOS. Calcium ion concentrations of 200- 400 nM in the central nervous system activate bNOS to catalyze the formation of NO. NO exerts definite effects on brain function although its specific roles are not well established. bNOS inhibitors have been found to block the release of neurotransmitter from presynaptic neurons. Excess levels of NO are also thought to contribute to neurodegenerative disorders such as Alzheimer's disease.
II. In the blood vessels, NO is produced by eNOS which is activated by Ca2+ concentrations of 200-400 nM. NO acts as the major endogenous vasodilator in blood vessels. It diffuses into smooth muscle cells and leads to muscle relaxation by stimulating cGMP formation through activation of guanylyl cyclase. In addition, NO regulates the vascular system by inhibiting platelet aggregation and adhesion.
III. The role of NO in the immune system is regulated by macNOS through a pathway that is not Ca2+ dependent. Rather, exposure to cytokines, including interleukin-1 and interferon- , leads to synthesis of large amounts of NO by activation of macNOS in response to inflammatory stimuli. The NO produced plays a definitive role in the mediation of the activities of macrophages and neutrophils. NO also acts to inhibit the mechanism of viral replication.
A "knock out" mouse with a mutant bNOS protein was generated by recombination techniques. The mutant protein was identical to the wild-type protein except for the identity of amino acid 675; the mutated bNOS has Tryptophan instead of Cysteine at position 675. Which of the following is responsible for the mutant protein?
A. Frame shift mutation
B. Single base-pair deletion
C. Point mutation
D. Nonsense mutation
-
Question 539:
Early experimentation on the single-celled organism Acetabularia led to important discoveries about the role of the nucleus in regulating cell function. Acetabularia is an enormous single cell with three distinct regions: a cap, a root-like rhizoid,
and a stalk which connects the two. The following experiments were conducted to study the development of the cell:
Experiment 1
The stalk of an Acetabularia was cut, fragmenting the cell. The fragment which included the cap died shortly afterwards while the fragment containing the rhizoid regenerated to form a complete Acetabularia.
Experiment 2
The nucleus from Acetabularia mediterranea, which has a flat cap, was transplanted into Acetabularia crenulata, which has a tufted cap, following removal of the Acetabularia crenulata nucleus. The Acetabularia crenulata cap eventually
assumed the flat shape.
Experiment 3
The nucleus of Acetabularia mediterranea was removed from the young cell before it first formed a cap. A normal cap formed several weeks later. The cell proved to be inviable and died shortly thereafter.
Experiment 4
A young Acetabularia was fractioned into a number of portions before it first formed a cap. Several weeks later, both the portion containing the nucleus and the portion containing the apical tip of the stalk formed caps. The other portions did
not form caps.
The mRNA sequence shown below is transcribed from which of the following DNA fragments?
z5'-UGUAAUC-3'
mRNA
A. 5'-GAUUACA-3'
B. 5'-ACATTAG-3'
C. 5'-CTAATGT-3'
D. 5'-GATTACA-3'
-
Question 540:
Nitric oxide, NO, has recently been found to have widespread physiological effects, acting as a major regulator in the nervous, cardiovascular, and immune systems. The production of NO in the body is regulated by specific NOS enzymes which exist in at least three different isoforms -- bNOS, eNOS, and macNOS. Each of these isoforms differ in location and function and serve to mediate different physiological responses to NO. Some physiological roles of NO have been demonstrated as follows:
I. In the central nervous system, NO production is regulated by bNOS. Calcium ion concentrations of 200- 400 nM in the central nervous system activate bNOS to catalyze the formation of NO. NO exerts definite effects on brain function although its specific roles are not well established. bNOS inhibitors have been found to block the release of neurotransmitter from presynaptic neurons. Excess levels of NO are also thought to contribute to neurodegenerative disorders such as Alzheimer's disease.
II. In the blood vessels, NO is produced by eNOS which is activated by Ca2+ concentrations of 200-400 nM. NO acts as the major endogenous vasodilator in blood vessels. It diffuses into smooth muscle cells and leads to muscle relaxation by stimulating cGMP formation through activation of guanylyl cyclase. In addition, NO regulates the vascular system by inhibiting platelet aggregation and adhesion.
III. The role of NO in the immune system is regulated by macNOS through a pathway that is not Ca2+ dependent. Rather, exposure to cytokines, including interleukin-1 and interferon- , leads to synthesis of large amounts of NO by activation of macNOS in response to inflammatory stimuli. The NO produced plays a definitive role in the mediation of the activities of macrophages and neutrophils. NO also acts to inhibit the mechanism of viral replication.
Based on information in the passage, which of the following is a possible effect of NO in the brain?
A. Inhibit the fusing of neurotransmitter vesicles at the presynaptic membrane
B. Promote neurotransmitter release into the synaptic cleft
C. Enhance vasoconstriction to maintain blood pressure
D. Decrease neurotransmitter synthesis by the presynaptic neuron
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.