Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Mar 30, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 51:
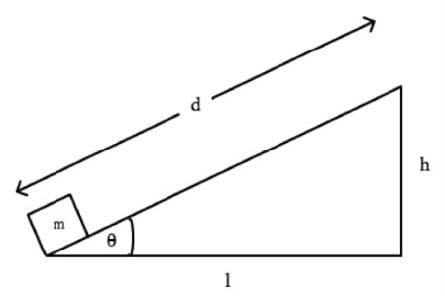
Which of the following expressions best approximates the work required to move the box with a mass m to the top of the ramp? (Assume that the ramp is frictionless.)
A. mglcosT
B. mghsinT
C. mgdcosT
D. mgdsinT
-
Question 52:
A source emits a sound from one medium with a certain velocity, intensity, frequency and wavelength. When the sound exits the first medium and enters a denser medium, all of the following changes EXCEPT:
A. velocity
B. intensity
C. frequency
D. wavelength
-
Question 53:
Arginine is one of the 20 most common natural amino acids. Most healthy people do not need to supplement with arginine because the body usually produces sufficient quantities. The pathway for arginine synthesis was studied using cells from a red bread mold. This natural form of arginine is illustrated below.
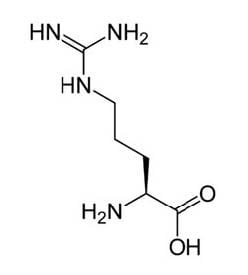
The red bread mold Neurospora crassa grows well on a cultural plate with "minimal" medium which is a fluid containing only a few simple sugars, inorganic salts, and vitamin. Neurospora that grows normally in nature (wild type) has enzymes that convert these simple substances into the amino acids necessary for growth. Mutating any one of the genes that makes an enzyme can produce a Neurospora strain that cannot grow on minimal medium. The mutant would only grow if the enzyme product were to be added as a supplement. On the other hand, if a "complete" medium is provided, containing all required amino acids, then Neurospora would grow, with or without mutation.

Figure 1 A synthesis pathway for the amino acid arginine. Each gene in italics in the diagram produces one enzyme necessary for the synthesis of this essential amino acid required for growth.
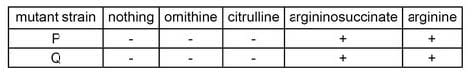
Table 1 Growth response of mutant strains in "minimal" media with supplements (ornithine, citrulline, argininosuccinate, and arginine) as indicated. Strain growth is indicated by (+) and no strain growth is indicated by (-). Which of the following is NOT an accurate description of naturally-occurring arginine?
A. Acidic amino acid
B. L-Configuration
C. -Amino acid
D. S-Configuration
-
Question 54:
In an SDS-PAGE procedure, the SDS serves as a detergent.
Why are the proteins treated with a detergent before being run through the electrophoresis gel?
A. To coat the proteins with a large positive charge, since amino acid side chains may have positive, negative, or neutral charges, and a large uniform charge is necessary to get good separation in the gel.
B. To allow the electrophoresis to separate the proteins solely on the basis of the length of the primary sequence.
C. To prevent the protein from denaturing so that the electrophoresis can accurately resolve the proteins on the basis of tertiary structure.
D. To break the intramolecular bonds holding the tertiary and primary structure of the protein together, thereby generating linear fragments that may be sorted on size.
-
Question 55:
Triacylglycerides consist of:
I) a ribose backbone II) a glycerol backbone III) three phosphodiester linkages IV) three ester linkages
A. I and III
B. II only
C. II and III
D. II and IV
-
Question 56:
Which of the following molecules is a hormone capable of creating physiological and anatomical changes during adolescence which will last a person's entire life?
A.
B.
C.
D.
-
Question 57:
The combination of the cornea and the crystalline lens shown below serves as a converging lens.
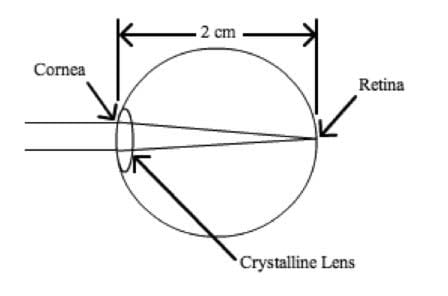
In a perfectly functioning eye, an image is projected at a fixed distance on the retina, which is approximately 2cm from the lens. What is the power of the converging lens when a person without any visual impairment stares at a distant object?
A. 0.5 diopters
B. 10 diopters
C. 25 diopters
D. 50 diopters
-
Question 58:
Assuming the circulatory system in humans obeys Bernoulli's principle of fluid dynamics, which of the statements most accurately compares the blood pressure in a capillary of the neck to a capillary with an equal cross-sectional area in the right knee?
A. The pressure in the neck is greater than the pressure in the knee because of the increase in pressure head.
B. The pressure in the neck is equal to the pressure in the knee because of the equal dynamic pressure according to the continuity equation.
C. The pressure in the knee is greater than the pressure in the neck because of the increase in pressure head.
D. An accurate comparison cannot be given without knowledge of the fluid's density and viscosity.
-
Question 59:
For a very weak base, the pKb of a solution would likely be:
A. Equal to the pOH
B. Higher than the pOH
C. Lower than the pOH
D. Near 7 at 25篊
-
Question 60:
A thin layer chromatography is performed on both the reactants and products of a reaction. It is found that the products have an Rf value that is significantly higher than the reactants.
Which of the following could adequately describe this reaction?
A. SN2 reaction converting an alkyl bromide to an alkyl chloride
B. Addition reaction converting an alkene to an alcohol
C. Nucleophilic acyl substitution reaction converting an ester to an anhydride
D. Elimination reaction converting an alcohol to an alkene
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.