Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Mar 30, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 61:
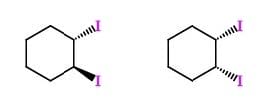
The two molecules above can best be described as:
A. Structural isomers
B. Diastereomers
C. Enantiomers
D. Conformational isomers
-
Question 62:
Mg(OH)2 is slowly dissolved in 500 mL of 25oC water until the solution becomes fully saturated. Which of the following occurs when 10.0 mL of 0.1 M HCl is added?
A. MgCl2 precipitates.
B. Mg(OH)2 precipitates.
C. Ksp for Mg(OH)2 increases.
D. [H2O] increases.
-
Question 63:
Which of the following best accounts for the negative slope of the liquid-solid equilibrium line in the phase diagram for water?
A. H2O(s) has a greater density than H2O(l), which causes the solid to form liquid under high pressure conditions.
B. H2O(s) has a greater density than H2O(l), which results from the hydrogen bonds formed between water molecules.
C. H2O(s) has a lower density than H2O(l) which results from the crystalline framework that forms due to hydrogen bonds.
D. H2O(s) has a lower density than H2O(l) which causes the solid to form liquid under low pressure conditions.
-
Question 64:
If a gas occupies 0.1L at 200atm, what will its volume be at 1atm?
A. slightly less than 20L
B. 20L
C. slightly more than 20L
D. 2000L
-
Question 65:
In an experiment to study frictional forces, a student attached a spring scale to identical bricks wrapped in different kinds of paper: brown paper, waxed paper and sand paper. Holding the spring scale, the student pulled the bricks across a table and measured the force required to pull the bricks across the table. The student wrapped the bricks in different paper to change the frictional forces between which two objects?
A. The bricks and the spring scale.
B. The paper and the bricks.
C. The wrapped bricks and the table.
D. None of these.
-
Question 66:
If KE =絤v2, express the unit of KE using the SI units of the terms in the equation.
A. kg2 x m2 x s-2
B. kg x m-2 x s2
C. kg x m2 x s-2
D. kg x m x s
-
Question 67:
If (sin[0] + cos[180]) = x - 2(sin[90]) and all angles are in degrees, what is x?
A. 0
B. -1
C. -2
D. 1
-
Question 68:
What happens to brain waves as an organism falls asleep?
A. The frequency increases and amplitude increases.
B. The frequency decreases and amplitude remains constant.
C. The frequency increases and amplitude decreases.
D. The frequency decreases and the amplitude increases.
-
Question 69:
A student observes that mercury forms a convex meniscus in the graduated cylinder but that water forms a concave one. This behavior is best explained by the fact that:
A. the two liquids are being kept in graduated cylinders made of different materials.
B. the adhesive forces between water and the walls of the graduated cylinder are greater than the adhesive forces between the mercury and the walls of the graduated cylinder.
C. the cohesive forces between two mercury atoms are stronger than the cohesive forces between two water molecules.
D. the mercury has strong cohesive than adhesive forces, whereas water has strong adhesive than cohesive ones.
-
Question 70:
What is the identity of the reducing agent and oxidizing agent, respectively, in the reaction shown below?
2Cr(OH)3 + 3H2O2 + 4NaOH 2Na2CrO4 + 8H2O
A. Cr(OH)+ and NaOH
B. Cr(OH)3 and H2O2
C. H2O2 and Cr(OH)3
D. H2O2 and NaOH
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.