Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 601:
Electromagnetic radiation from space constantly bombards the earth. Most wavelengths are absorbed by the atmosphere; however, there are two "windows" of nonabsorption through which significant amounts of radiation reach the ground. The first transmits ultraviolet and visible light, as well as infrared light or heat; the second transmits radio waves. As a result, terrestrial organisms have evolved a number of pigments that interact with light in various ways: some capture light energy, some provide protection from light- induced damage, and some serve camouflage or signaling purposes.
Among these compounds are many conjugated polyenes, which play important roles as photoreceptors. For every chemical compound, there are certain wavelengths of light whose quanta possess exactly the correct amount of energy to raise electrons from their ground state to higher-energy orbitals. For most organic compounds, these wavelengths are in the UV range. However, conjugated double bond systems stabilize the electrons, so that they can be excited by lower-frequency photons with wavelengths in the visible spectrum. Such a pigment, known as a chromophore, will then transmit the "subtraction color," a color complementary to the one absorbed. For instance, carotene, a hydrocarbon compound with eleven conjugated double bonds, absorbs blue light and transmits orange. The wavelength that is absorbed generally increases with the number of conjugated bonds; rings and side-chains also affect wavelength.
Wavelength Color Subtraction Color 480 nm blue orange 580 nm yellow violet 680 nm red green
Among the many biological molecules that are affected by light is DNA, the genetic material of living organisms. DNA absorbs ultraviolet light, and may be damaged by UVC (< 280 nm) and UVB (280-315 nm). UVA (315-400 nm) and visible light can actually repair light-induced damage to DNA by a process called photorepair. For this reason, UVA, which also stimulates tanning, was once considered beneficial. However, there is now increasing evidence that UVA can damage skin.
Many crustaceans produce a blue or green caroteneprotein complex. What is the most likely cause of the color change from green to orange when a lobster is boiled?
A. Heat causes the prosthetic group to become partially hydrated.
B. The increase in temperature permits the prosthetic group to absorb shorter wavelengths.
C. The protein is separated from the carotenoid pigment.
D. Heat causes the prosthetic group to become oxidized.
-
Question 602:
Electromagnetic radiation from space constantly bombards the earth. Most wavelengths are absorbed by the atmosphere; however, there are two "windows" of nonabsorption through which significant amounts of radiation reach the ground.
The first transmits ultraviolet and visible light, as well as infrared light or heat; the second transmits radio waves. As a result, terrestrial organisms have evolved a number of pigments that interact with light in various ways: some capture light
energy, some provide protection from light- induced damage, and some serve camouflage or signaling purposes.
Among these compounds are many conjugated polyenes, which play important roles as photoreceptors. For every chemical compound, there are certain wavelengths of light whose quanta possess exactly the correct amount of energy to
raise electrons from their ground state to higher-energy orbitals. For most organic compounds, these wavelengths are in the UV range. However, conjugated double bond systems stabilize the electrons, so that they can be excited by lower-
frequency photons with wavelengths in the visible spectrum. Such a pigment, known as a chromophore, will then transmit the "subtraction color," a color complementary to the one absorbed. For instance, carotene, a hydrocarbon compound
with eleven conjugated double bonds, absorbs blue light and transmits orange. The wavelength that is absorbed generally increases with the number of conjugated bonds; rings and side-chains also affect wavelength.
Wavelength Color Subtraction Color
480 nm blue orange
580 nm yellow violet
680 nm red green
Among the many biological molecules that are affected by light is DNA, the genetic material of living organisms. DNA absorbs ultraviolet light, and may be damaged by UVC (< 280 nm) and UVB (280-315 nm). UVA (315-400 nm) and visible
light can actually repair light-induced damage to DNA by a process called photorepair. For this reason, UVA, which also stimulates tanning, was once considered beneficial. However, there is now increasing evidence that UVA can damage
skin.
Why is benzene colorless?
A. The absorption energy is of too high a frequency to be visible.
B. The absorption energy is of too low a frequency to be visible.
C. Benzene does not absorb light.
D. Benzene is not conjugated.
-
Question 603:
Electromagnetic radiation from space constantly bombards the earth. Most wavelengths are absorbed by the atmosphere; however, there are two "windows" of nonabsorption through which significant amounts of radiation reach the ground. The first transmits ultraviolet and visible light, as well as infrared light or heat; the second transmits radio waves. As a result, terrestrial organisms have evolved a number of pigments that interact with light in various ways: some capture light energy, some provide protection from light- induced damage, and some serve camouflage or signaling purposes.
Among these compounds are many conjugated polyenes, which play important roles as photoreceptors. For every chemical compound, there are certain wavelengths of light whose quanta possess exactly the correct amount of energy to raise electrons from their ground state to higher-energy orbitals. For most organic compounds, these wavelengths are in the UV range. However, conjugated double bond systems stabilize the electrons, so that they can be excited by lower-frequency photons with wavelengths in the visible spectrum. Such a pigment, known as a chromophore, will then transmit the "subtraction color," a color complementary to the one absorbed. For instance, carotene, a hydrocarbon compound with eleven conjugated double bonds, absorbs blue light and transmits orange. The wavelength that is absorbed generally increases with the number of conjugated bonds; rings and side-chains also affect wavelength.
Wavelength Color Subtraction Color 480 nm blue orange 580 nm yellow violet 680 nm red green Among the many biological molecules that are affected by light is DNA, the genetic material of living organisms. DNA absorbs ultraviolet light, and may be damaged by UVC (< 280 nm) and UVB (280-315 nm). UVA (315-400 nm) and visible light can actually repair light-induced damage to DNA by a process called photorepair. For this reason, UVA, which also stimulates tanning, was once considered beneficial. However, there is now increasing evidence that UVA can damage skin.
Two pigments are identical except for the lengths of their conjugated polyene chains. The first transmits yellow light and the second red. What can be said about the sizes of the chromophores?
A. The first is longer.
B. The second is longer.
C. One of the chromophores must be a dimer.
D. The comparative lengths cannot be determined.
-
Question 604:
Electromagnetic radiation from space constantly bombards the earth. Most wavelengths are absorbed by the atmosphere; however, there are two "windows" of nonabsorption through which significant amounts of radiation reach the ground. The first transmits ultraviolet and visible light, as well as infrared light or heat; the second transmits radio waves. As a result, terrestrial organisms have evolved a number of pigments that interact with light in various ways: some capture light energy, some provide protection from light- induced damage, and some serve camouflage or signaling purposes.
Among these compounds are many conjugated polyenes, which play important roles as photoreceptors. For every chemical compound, there are certain wavelengths of light whose quanta possess exactly the correct amount of energy to raise electrons from their ground state to higher-energy orbitals. For most organic compounds, these wavelengths are in the UV range. However, conjugated double bond systems stabilize the electrons, so that they can be excited by lower-frequency photons with wavelengths in the visible spectrum. Such a pigment, known as a chromophore, will then transmit the "subtraction color," a color complementary to the one absorbed. For instance, carotene, a hydrocarbon compound with eleven conjugated double bonds, absorbs blue light and transmits orange. The wavelength that is absorbed generally increases with the number of conjugated bonds; rings and side-chains also affect wavelength.
Wavelength Color Subtraction Color 480 nm blue orange 580 nm yellow violet 680 nm red green
Among the many biological molecules that are affected by light is DNA, the genetic material of living organisms. DNA absorbs ultraviolet light, and may be damaged by UVC (< 280 nm) and UVB (280-315 nm). UVA (315-400 nm) and visible light can actually repair light-induced damage to DNA by a process called photorepair. For this reason, UVA, which also stimulates tanning, was once considered beneficial. However, there is now increasing evidence that UVA can damage skin.
The electrons that give color to a carotene molecule are found in:
A. s orbitals.
B. orbitals.
C. d orbitals.
D. f orbitals.
-
Question 605:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.
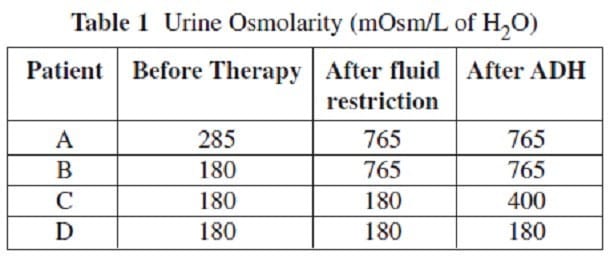
What is the most likely cause of Patient B's dilute urine before therapy?
A. Excessive water intake
B. Dehydration
C. Nephrogenic DI
D. Central DI
-
Question 606:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.

Based on the data in Table 1, which of the four patients most likely has nephrogenic diabetes insipidus?
A. Patient A
B. Patient B
C. Patient C
D. Patient D
-
Question 607:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.

Based on the data in Table 1, which of the four patients most likely has central diabetes insipidus?
A. Patient A
B. Patient B
C. Patient C
D. Patient D
-
Question 608:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.

Which of the following would you most likely expect to find in a patient with diabetes insipidus?
A. Decreased plasma osmolarity
B. Increased urine osmolarity
C. Increased urine glucose
D. Increased urine output
-
Question 609:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.

Which of the following substances would NOT be found in appreciable quantity in the urine of a healthy individual?
A. Albumin
B. Sodium
C. Urea
D. Potassium
-
Question 610:
Just as the ingestion of nutrients is mandatory for human life, so is the excretion of metabolic waste products. One of these nutrients, protein, is used for building muscle, nucleic acids, and countless compounds integral to homeostasis. However, the catabolism of the amino acids generated from protein digestion produces ammonia, which, if not further degraded, can become toxic. Similarly, if the same salts that provide energy and chemical balance to cells are in excess, fluid retention will occur, damaging the circulatory, cardiac, and pulmonary systems.
One of the most important homeostatic organs is the kidney, which closely regulates the excretion and reabsorption of many essential ions and molecules. One mechanism of renal function involves the secretion of antidiuretic hormone (ADH).
Diabetes insipidus (DI), is the condition that occurs when ADH is ineffective. As a result, the kidneys are unable to concentrate urine, leading to excessive water loss. There are two types of DI -- central and nephrogenic. Central DI occurs when there is a deficiency in the quantity or quality of ADH produced. Nephrogenic DI occurs when the kidney tubules are unresponsive to ADH. To differentiate between these two conditions, a patient's urine osmolarity is measured both prior to therapy and after a 24-hour restriction on fluid intake. Exogenous ADH is then administered and urine osmolarity is measured again. The table below gives the results of testing on four patients. Assume that a urine osmolarity of 285 mOsm/L of H2O is normal.

According to the passage, the catabolism of amino acids produces ammonia. Therefore, after a proteinrich meal, would you expect a build-up of ammonia in the lumen of the small intestine?
A. Yes, because the ammonia will not be able to diffuse into the intestinal epithelium.
B. Yes, because the rate at which digestive enzymes degrade ammonia is slower than the rate at which ammonia is produced.
C. No, because the ammonia will diffuse into the intestinal epithelium and will be excreted by the kidneys.
D. No, because the ammonia is produced inside individual cells, not within the lumen of the small intestine.
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.