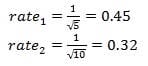Exam Details
Exam Code
:PCATExam Name
:Pharmacy College Admission TestCertification
:Admission Tests CertificationsVendor
:Admission TestsTotal Questions
:282 Q&AsLast Updated
:Apr 11, 2025
Admission Tests Admission Tests Certifications PCAT Questions & Answers
-
Question 131:
What would be the percentage mass of chlorine in a molecule of methyl chloride? (C = 12 gm/mole, H = 1 gm/mole, Cl = 35.5 gm/mole).
A. 18 %
B. 50 %
C. 70 %
D. 12 %
-
Question 132:
The region in space where an electron is likely to be found is called a(n):
A. Axis
B. Cloud
C. Orbital D. Configuration
-
Question 133:
The rate law for a reaction is of the second order. Which statement is true?
A. The rate must depend on both reactants.
B. The reaction must depend on the square of one reactant.
C. The reaction must depend on only k squared.
D. The reaction must depend on at least one of the reactants.
-
Question 134:
Which of the following has the highest boiling point?
A. methanol
B. n-propanol
C. isopropanol
D. ethanol
-
Question 135:
What is the result of adding HBr and hydrogen peroxide to propene?
A. 2-propanol
B. 1-propanol
C. 2-bromopropane
D. 1-bromopropane
-
Question 136:
Rank the following amino acids by increasing pI (isoelectric point):
I. Lys
II. Leu
III.
Asp
A.
I < II < III
B.
III < II < I
C.
II < I < III
D.
III < I < II
-
Question 137:
Which effect would result from increasing the molar mass of a gas?
A. An increase in temperature
B. An increase on pressure
C. An increase in volume
D. A decrease in the rate
-
Question 138:
Which of the following species cannot hydrogen bond with itself?
A. ethanol
B. acetic acid
C. ammonia
D. acetone
-
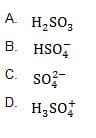
Question 139:
What is the conjugate base of sulfuric acid?
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 140:
What would be the result of increasing the number of nucleophiles in a SN1 reaction?
A. It would increase the rate exponentially.
B. It would increase the rate linearly.
C. It would decrease the rate.
D. It would have no effect.
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Admission Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your PCAT exam preparations and Admission Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.