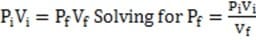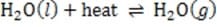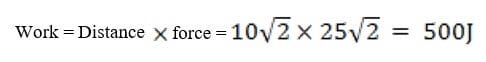Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 171:
The nuclei of certain unstable isotopes will spontaneously decay, producing a more stable nucleus and releasing a particle or quantity of energy. Alpha decay releases a helium nucleus, beta decay emits an electron, while gamma decay is the emission of a high energy photon. Each type of radioactive decay is characterized, in part, by the half-life of the radioactive material--the time required for half of the nuclei in a sample to undergo decay. Examples of such decays are shown in Figure 1.
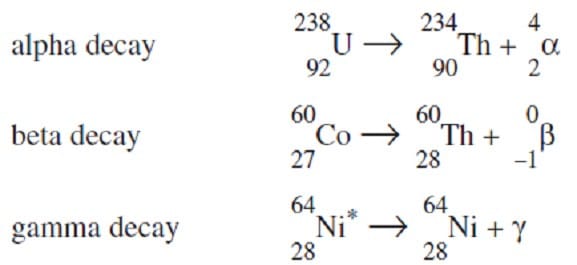
Figure 1
A Geiger counter can be used to detect the decay of radioactive materials. A simple Geiger counter consists of a hollow metal cylinder with a wire along its axis. The cylinder is filled with low pressure argon gas and a high voltage difference is
applied between the wire and the cylinder. When alpha, beta, or gamma radiation passes through the cylinder, it interacts with the gas particles and leads to the formation of ions which cause a discharge between the wire and the cylinder.
The consequent current may be used to drive a speaker, producing the characteristic clicking sound of the Geiger counter each time a pulse of current occurs. The Geiger counter circuitry is shown in Figure 2.
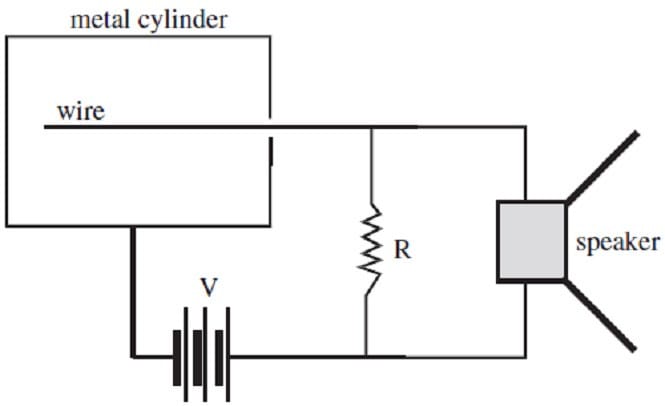
Figure 2
Assume that the values for V and R are known. What other information is needed to calculate the voltage drop across the speaker?
A. The resistance of the speaker's internal circuitry.
B. The duration of the discharge between the wire and the cylinder.
C. The current flowing through the resistor.
D. No other information is required.
-
Question 172:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.
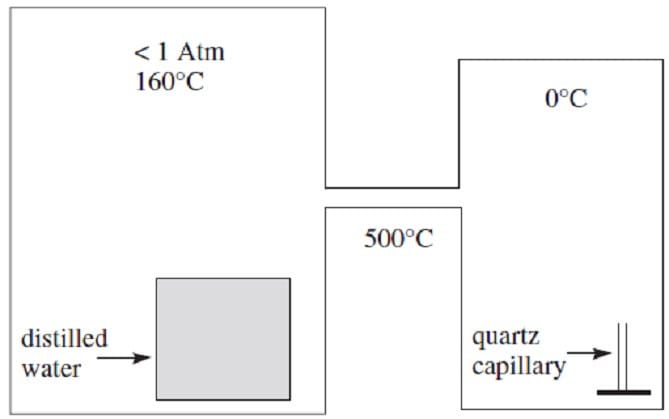
Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.
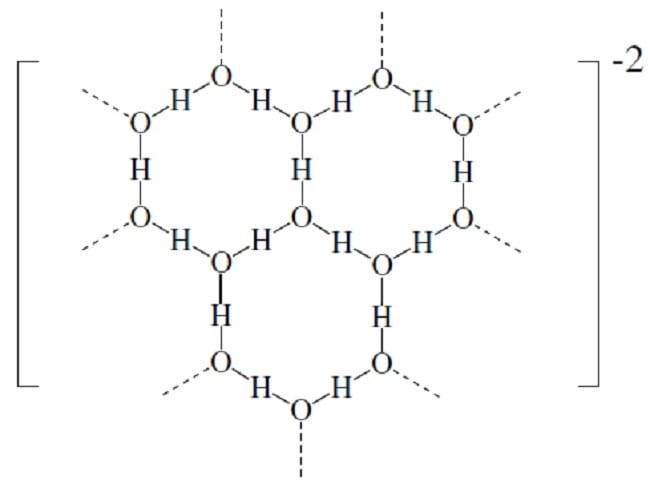
Proposed Structure of Polywater Hypothesis 2
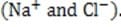
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )
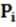
The pressure ( ) in the first chamber is decreased by raising a piston and increasing the volume of the chamber from to . If the temperature is kept at 160 °C, which of the following values solves for the new pressure ( ) in the chamber?
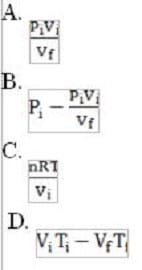
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 173:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2 Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.
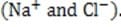

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )
Which of the following would be most likely to occur if the second chamber was kept at 50?C instead of 0?C?
A. The water vapor would not condense.
B. Liquid water would be able to travel through the tube between chambers.
C. A different polymer of polywater would form.
D. The water vapor would condense more slowly.
-
Question 174:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48. An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )
Which of the following pieces of evidence would most support Hypothesis 1?
A. The mass of the quartz capillary did not change throughout the experiment.
B. Filtration of the polywater increased its freezing temperature.
C. The polywater was found to differ from normal water in its boiling point.
D. The second chamber could be kept at 50?C with similar results.
-
Question 175:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )
Which of the following changes in the experimental apparatus would increase the rate of water vapor production in the first chamber of the experimental apparatus? (All other conditions kept constant.)
A. Decreasing the temperature
B. Decreasing the pressure
C. Decreasing the cross-sectional area of the tube connecting the two chambers
D. Adding NaCl to the distilled water in the first chamber
-
Question 176:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )
Assume that Hypothesis 2 is correct. Compared to normal water, polywater would have a:
A. lower vapor pressure and higher boiling point.
B. lower vapor pressure and lower boiling point.
C. higher vapor pressure and lower boiling point.
D. similar vapor pressure and boiling point.
-
Question 177:
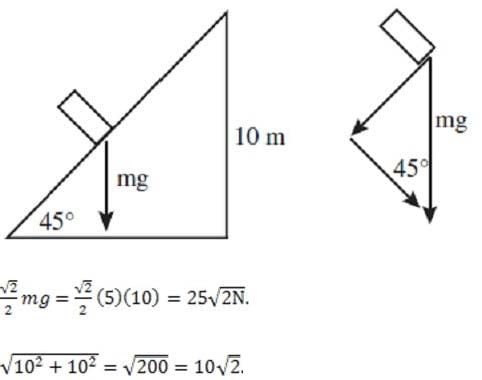
A5-kg mass M is being raised from the ground to the top of the inclined plane using the set-up shown in the diagram below. Assuming that the inclined plane is frictionless, what is the work done by the force F?
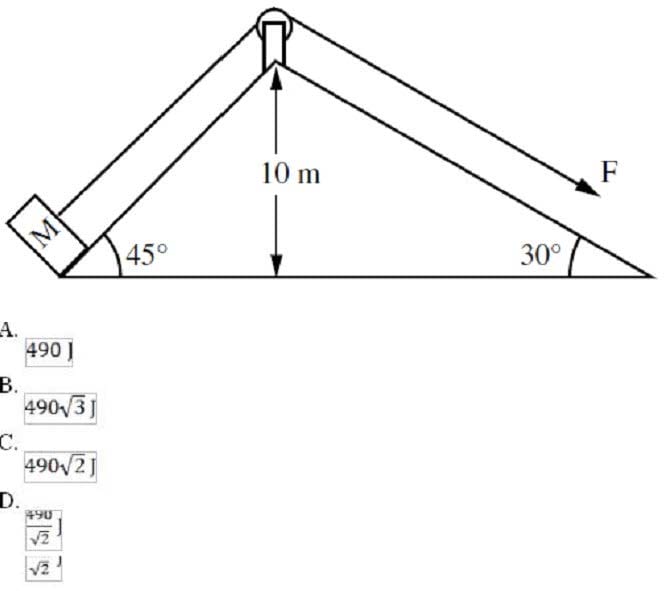
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 178:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C )

Hypothesis 1 proposes that polywater is a repeating structure of monomers. What is the formal charge on hydrogen in this structure?
A. -1
B. +1
C. -2
D. +2
-
Question 179:
In 1965, Boris Deryagin reported the discovery of an unusual substance formed during the condensation of water vapor in quartz capillaries. The material, called poly-water, appeared to be a polymer of water monomers and differed from normal water in a number of ways. It had a freezing point of ?0?C and solidified into a glass-like solid with substantially less volumetric expansion than that of ordinary water upon freezing. It had a density 40% greater than water and a refractive index of 1.48.
An intricate apparatus was used to produce the poly-water. Ordinary distilled water was placed in a chamber held at 160?C with pressure below atmospheric pressure. This chamber was connected to a second chamber by a tube held at 500?C in order to prevent the passage of liquid water. The second chamber was held at 0?C and contained a drawn quartz capillary in which the water vapor condensed, forming poly-water.

Hypothesis 1
Deryagin proposed that polywater was a polymer of water monomers arranged in a network of hexagonal units. The polymerization was catalyzed by the silicate surface of the quartz capillary.

Proposed Structure of Polywater Hypothesis 2
Another researcher was skeptical. Analysis indicated that polywater was merely a solution of water and dissolved particles including silicon, carbon dioxide, and substantial concentrations of ions These contaminants dissolved from the quartz capillary and from materials used in the apparatus.

(constants for normal water : density = 1 g/c , index of refraction = 1.33 , freezing point depression constant = 1.86°C ) According to the passage, polywater has all of the following properties EXCEPT:
A. light entering polywater from air will bend more towards the normal than light entering normal water.
B. polywater can be produced in a variety of conditions.
C. 1 g of water has a larger volume than 1 g of polywater, at room temperature.
D. polywater exists in the liquid state at 0?C.
-
Question 180:
A student conducts a chemical analysis of the components of a popular soft drink. The beverage label shows that the drink contains carbonated water, phosphoric acid, caffeine, and caramel color, but does not indicate the concentrations of these chemicals.
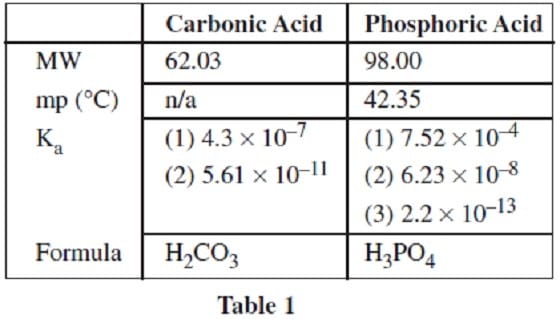
Dissolved carbon dioxide will react reversibly with water to form carbonic acid. In an attempt to analyze the beverage composition, the student conducts the following experiments on a one liter sample of the beverage.
Experiment 1
The sample is placed in a sealed beaker cooled to 10?C and a vacuum is created in the space above the beverage. The gas pumped from this space is passed through a solution of BaCl2, producing a white precipitate. The process
continues until no more precipitate forms. The precipitate is dried and found to have a mass of 9.5 grams.
Experiment 2
The remaining solution left in the sealed beaker is then titrated with 0.01 M NaOH to give the titration curve shown in Figure 1.

Figure 1
Why did the student choose to keep the sealed beaker of beverage at 10?C while vacuuming the from solution?

A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.