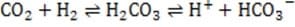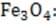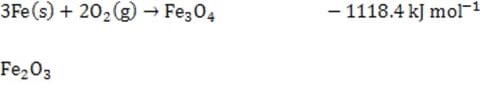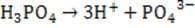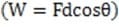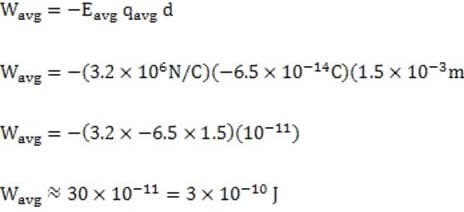Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 181:
A student conducts a chemical analysis of the components of a popular soft drink. The beverage label shows that the drink contains carbonated water, phosphoric acid, caffeine, and caramel color, but does not indicate the concentrations of these chemicals.
Dissolved carbon dioxide will react reversibly with water to form carbonic acid. In an attempt to analyze the beverage composition, the student conducts the following experiments on a one liter sample of the beverage.
Experiment 1
The sample is placed in a sealed beaker cooled to 10?C and a vacuum is created in the space above the beverage. The gas pumped from this space is passed through a solution of BaCl2, producing a white precipitate. The process
continues until no more precipitate forms. The precipitate is dried and found to have a mass of 9.5 grams.
Experiment 2
The remaining solution left in the sealed beaker is then titrated with 0.01 M NaOH to give the titration curve shown in Figure 1.

Figure 1
In the body, rapid breathing (hyperventilation) leads to a decrease in the concentration of carbon dioxide in the blood. This will tend to:

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 182:
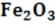
Given the following H?values, calculate the H?of formation of (s).


A. Option A
B. Option B
C. Option C
D. Option D
-
Question 183:
Ink jet printers produce high resolution output, at a lower cost than laser printers, by generating charged ink droplets which are then deflected onto a sheet of paper by an electric field. Each droplet deflected by the field strikes the paper and forms a tiny dot of ink. While a typical printed letter requires about 100 drops, an ink jet printer is able to produce drops at a rate of 100,000 per second.
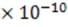
The essential elements of the ink jet printer head are shown in Figure 1. The drop generator produces the ink droplets, each with a mass of approximately 1.2 kg and a diameter of approximately 30 m. The drops then enter a highly precise charging unit which controls the charge q on each droplet to within 2%, with typical charges for drops generated by various ink jet printers ranging from ?.0 C to ?.0 C. The charged droplets are
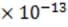
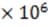
subsequently passed through the deflecting plates between which a variable electric field is generated. The electronically controlled electric field between the plates is typically varied over a range from 1.0 N/C to 5.4 N/C,
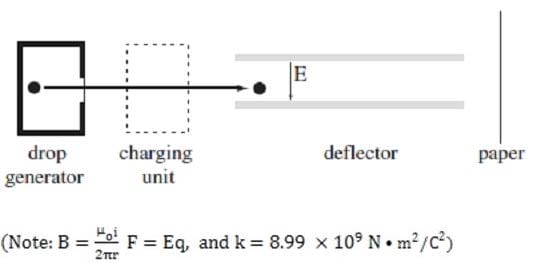
and is used to aim the ink droplet at the paper. .
In order to observe the velocity of the ink droplet, a magnetic field is generated to exactly counter the force applied to the ink droplet by the electric field. This magnetic field is oriented:
A. towards the left.
B. towards the right.
C. out of the page.
D. into the page.
-
Question 184:
A student conducts a chemical analysis of the components of a popular soft drink. The beverage label shows that the drink contains carbonated water, phosphoric acid, caffeine, and caramel color, but does not indicate the concentrations of these chemicals.

Dissolved carbon dioxide will react reversibly with water to form carbonic acid. In an attempt to analyze the beverage composition, the student conducts the following experiments on a one liter sample of the beverage.
Experiment 1
The sample is placed in a sealed beaker cooled to 10?C and a vacuum is created in the space above the beverage. The gas pumped from this space is passed through a solution of BaCl2, producing a white precipitate. The process
continues until no more precipitate forms. The precipitate is dried and found to have a mass of 9.5 grams.
Experiment 2
The remaining solution left in the sealed beaker is then titrated with 0.01 M NaOH to give the titration curve shown in Figure 1.

Figure 1
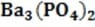
is completely insoluble in aqueous solution. Addition of barium chloride to a phosphoric acid solution will decrease the pH because:
A. barium acts as a Lewis base.
B. barium chloride reduces the concentration of hydrogen ions in solution.
C. chloride ion acts to stabilize the hydrogen ions in solution.
D. the precipitation of barium phosphate drives the acid dissociation to completion.
-
Question 185:
A student conducts a chemical analysis of the components of a popular soft drink. The beverage label shows that the drink contains carbonated water, phosphoric acid, caffeine, and caramel color, but does not indicate the concentrations of these chemicals.

Dissolved carbon dioxide will react reversibly with water to form carbonic acid. In an attempt to analyze the beverage composition, the student conducts the following experiments on a one liter sample of the beverage.
Experiment 1
The sample is placed in a sealed beaker cooled to 10?C and a vacuum is created in the space above the beverage. The gas pumped from this space is passed through a solution of BaCl2, producing a white precipitate. The process
continues until no more precipitate forms. The precipitate is dried and found to have a mass of 9.5 grams.
Experiment 2
The remaining solution left in the sealed beaker is then titrated with 0.01 M NaOH to give the titration curve shown in Figure 1.

Figure 1
Which region of the graph in Figure 1 provides the best buffering around neutral pH?
A. A
B. B
C. C
D. D
-
Question 186:
Ink jet printers produce high resolution output, at a lower cost than laser printers, by generating charged ink droplets which are then deflected onto a sheet of paper by an electric field. Each droplet deflected by the field strikes the paper and forms a tiny dot of ink. While a typical printed letter requires about 100 drops, an ink jet printer is able to produce drops at a rate of 100,000 per second.
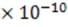
The essential elements of the ink jet printer head are shown in Figure 1. The drop generator produces the ink droplets, each with a mass of approximately 1.2 kg and a diameter of approximately 30 m. The drops then enter a
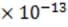
highly precise charging unit which controls the charge q on each droplet to within 2%, with typical charges for drops generated by various ink jet printers ranging from ?.0 C to ?.0 C. The charged droplets are
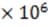
subsequently passed through the deflecting plates between which a variable electric field is generated. The electronically controlled electric field between the plates is typically varied over a range from 1.0 N/C to 5.4 N/C,
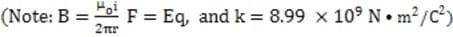
and is used to aim the ink droplet at the paper. .
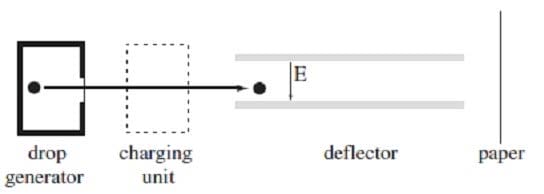
An ink jet printer deflects a particular ink droplet by 1.5 mm in the region of the deflector. Which of the following is a possible value of the work done on the droplet?
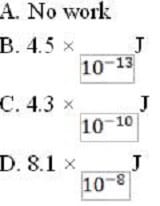
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 187:
Ink jet printers produce high resolution output, at a lower cost than laser printers, by generating charged ink droplets which are then deflected onto a sheet of paper by an electric field. Each droplet deflected by the field strikes the paper and forms a tiny dot of ink. While a typical printed letter requires about 100 drops, an ink jet printer is able to produce drops at a rate of 100,000 per second.
The essential elements of the ink jet printer head are shown in Figure 1. The drop generator produces the ink droplets, each with a mass of approximately 1.2 kg and a diameter of approximately 30 m. The drops then enter a
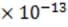
highly precise charging unit which controls the charge q on each droplet to within 2%, with typical charges for drops generated by various ink jet printers ranging from ?.0 C to ?.0 C. The charged droplets are
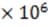
subsequently passed through the deflecting plates between which a variable electric field is generated. The electronically controlled electric field between the plates is typically varied over a range from 1.0 N/C to 5.4 N/C,

and is used to aim the ink droplet at the paper. .
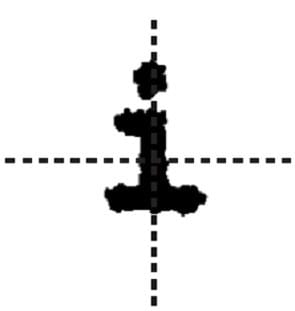
Suppose that an ink-jet printer head is programmed to produce the letter shown below (note that cross hairs are not produced by the ink jet printer head).
An uniform external electric field pointing upwards is superimposed on the printer head, and the letter is printed again. Which of the following diagrams best represents the new printer output?
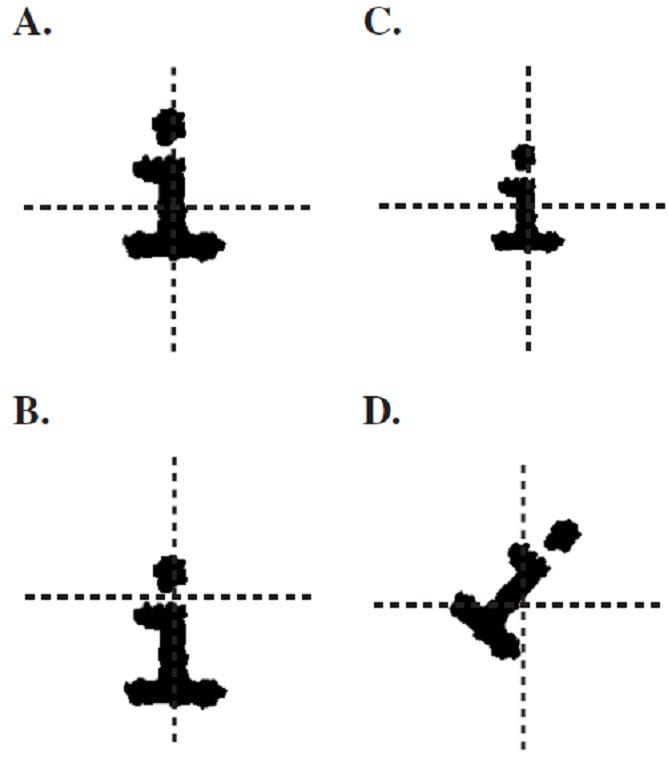
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 188:
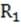
In the circuit below, = = 10 ohms. Removing will have which of the following effects on the current through ?
A. Decrease to 1/2
B. No change
C. Double
D. Increase by factor of 10
-
Question 189:
Ink jet printers produce high resolution output, at a lower cost than laser printers, by generating charged ink droplets which are then deflected onto a sheet of paper by an electric field. Each droplet deflected by the field strikes the paper and forms a tiny dot of ink. While a typical printed letter requires about 100 drops, an ink jet printer is able to produce drops at a rate of 100,000 per second.
The essential elements of the ink jet printer head are shown in Figure 1. The drop generator produces the ink droplets, each with a mass of approximately 1.2 kg and a diameter of approximately 30 m. The drops then enter a
highly precise charging unit which controls the charge q on each droplet to within 2%, with typical charges for drops generated by various ink jet printers ranging from ?.0 C to ?.0 C. The charged droplets are subsequently passed through the deflecting plates between which a variable electric field is generated. The electronically controlled electric field between the plates is typically varied over a range from 1.0 N/C to 5.4 N/C,
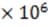
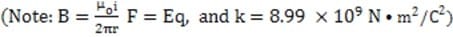
and is used to aim the ink droplet at the paper. .

In which direction would the deflector in Figure 1 deflect the ink drop?
A. Upwards in the plane of the page
B. Downwards in the plane of the page
C. Into the plane of the page
D. Out of the plane of the page
E. (0,1) and (2,1)
F. (0,1) and (0,2)
G. (1,1) and (3,1)
H. (2,1) and (1,2)
-
Question 190:
A 2-stage rocket is launched vertically upwards at velocity v. At height h it discards its first stage in four fragments and travels faster without changing direction. Which of the following views from above could be the velocity vectors of the first stage fragments?
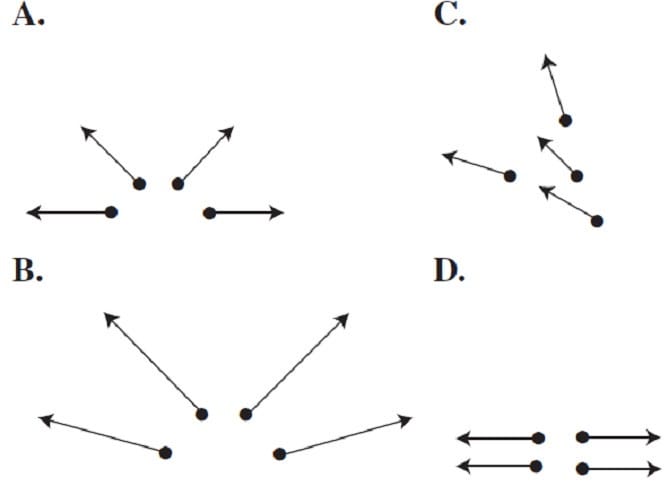
A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.