Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 261:
Band theory explains the conductivity of certain solids by stating that the atomic orbitals of the individual atoms in the solid merge to produce a series of atomic orbitals comprising the entire solid. The closely-spaced energy levels of the orbitals form bands. The band corresponding to the outermost occupied subshell of the original atoms is called the valence band. If partially full, as in metals, it serves as a conduction band through which electrons can move freely. If the valence band is full, then electrons must be raised to a higher band for conduction to occur. The greater the band gap between the separate valence and conduction bands, the poorer the material's conductivity. Figure 1 shows the valence and conduction bands of a semiconductor, which is intermediate in conductivity between conductors and insulators.
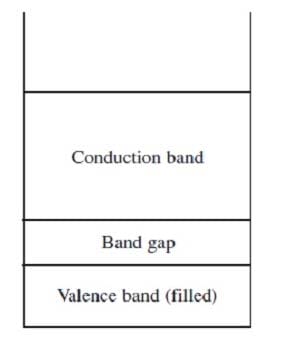
Figure 1
When silicon, a semiconductor with tetrahedral covalent bonds, is heated, a few electrons escape into the conduction band. Doping the silicon with a few phosphorus atoms provides unbonded electrons that escape more easily, increasing conductivity. Doping with boron produces holes in the bonding structure, which may be filled by movement of nearby electrons within the lattice. When a semiconductor in an electric circuit has excess electrons on one side and holes on the other, electron flow occurs more easily from the side with excess electrons to the side with holes than in the reverse direction.
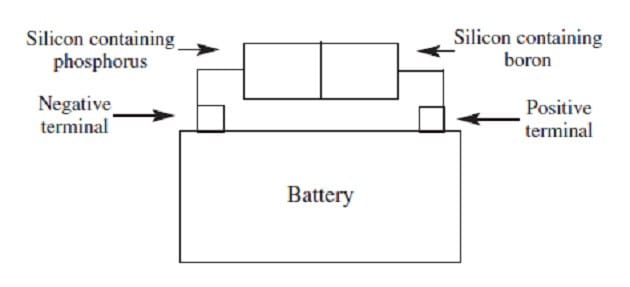
Figure 2
The energy of the band gap for pure silicon is about 1.1 electron volts. If a 1.5-volt electrical potential is connected across a sample of silicon:
A. the electrons would jump to the conduction band and the silicon would conduct.
B. the holes in the silicon lattice would move.
C. the energy of the band gap would be lowered.
D. the silicon would not conduct.
-
Question 262:
Band theory explains the conductivity of certain solids by stating that the atomic orbitals of the individual atoms in the solid merge to produce a series of atomic orbitals comprising the entire solid. The closely-spaced energy levels of the orbitals form bands. The band corresponding to the outermost occupied subshell of the original atoms is called the valence band. If partially full, as in metals, it serves as a conduction band through which electrons can move freely. If the valence band is full, then electrons must be raised to a higher band for conduction to occur. The greater the band gap between the separate valence and conduction bands, the poorer the material's conductivity. Figure 1 shows the valence and conduction bands of a semiconductor, which is intermediate in conductivity between conductors and insulators.
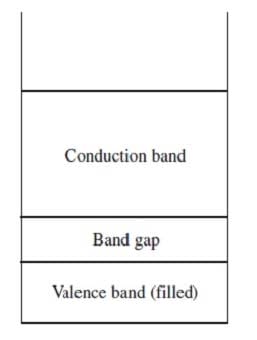
Figure 1
When silicon, a semiconductor with tetrahedral covalent bonds, is heated, a few electrons escape into the conduction band. Doping the silicon with a few phosphorus atoms provides unbonded electrons that escape more easily, increasing conductivity. Doping with boron produces holes in the bonding structure, which may be filled by movement of nearby electrons within the lattice. When a semiconductor in an electric circuit has excess electrons on one side and holes on the other, electron flow occurs more easily from the side with excess electrons to the side with holes than in the reverse direction.
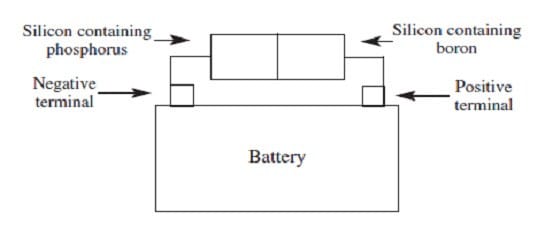
Figure 2
Why do phosphorus and boron atoms enhance the conductivity of silicon?
A. Their electronegativities differ from that of silicon.
B. They have different numbers of valence electrons.
C. Their semimetallic nature makes them good semiconductors.
D. They are better conductors than silicon even as pure substances.
-
Question 263:
Band theory explains the conductivity of certain solids by stating that the atomic orbitals of the individual atoms in the solid merge to produce a series of atomic orbitals comprising the entire solid. The closely-spaced energy levels of the orbitals form bands. The band corresponding to the outermost occupied subshell of the original atoms is called the valence band. If partially full, as in metals, it serves as a conduction band through which electrons can move freely. If the valence band is full, then electrons must be raised to a higher band for conduction to occur. The greater the band gap between the separate valence and conduction bands, the poorer the material's conductivity. Figure 1 shows the valence and conduction bands of a semiconductor, which is intermediate in conductivity between conductors and insulators.
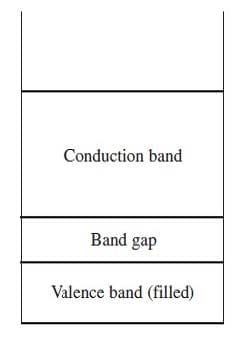
Figure 1
When silicon, a semiconductor with tetrahedral covalent bonds, is heated, a few electrons escape into the conduction band. Doping the silicon with a few phosphorus atoms provides unbonded electrons that escape more easily, increasing conductivity. Doping with boron produces holes in the bonding structure, which may be filled by movement of nearby electrons within the lattice. When a semiconductor in an electric circuit has excess electrons on one side and holes on the other, electron flow occurs more easily from the side with excess electrons to the side with holes than in the reverse direction.

Figure 2
Why is iron a good conductor of electricity?
A. Its 3d electrons only partially fill the valence band.
B. The band gap is small.
C. The 4s and 3d orbitals form a filled valence band.
D. The energy levels of the atomic orbitals are closely separated.
-
Question 264:
Band theory explains the conductivity of certain solids by stating that the atomic orbitals of the individual atoms in the solid merge to produce a series of atomic orbitals comprising the entire solid. The closely-spaced energy levels of the orbitals form bands. The band corresponding to the outermost occupied subshell of the original atoms is called the valence band. If partially full, as in metals, it serves as a conduction band through which electrons can move freely. If the valence band is full, then electrons must be raised to a higher band for conduction to occur. The greater the band gap between the separate valence and conduction bands, the poorer the material's conductivity. Figure 1 shows the valence and conduction bands of a semiconductor, which is intermediate in conductivity between conductors and insulators.
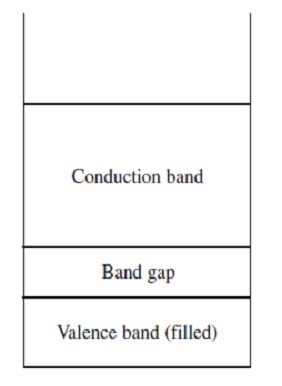
Figure 1
When silicon, a semiconductor with tetrahedral covalent bonds, is heated, a few electrons escape into the conduction band. Doping the silicon with a few phosphorus atoms provides unbonded electrons that escape more easily, increasing conductivity. Doping with boron produces holes in the bonding structure, which may be filled by movement of nearby electrons within the lattice. When a semiconductor in an electric circuit has excess electrons on one side and holes on the other, electron flow occurs more easily from the side with excess electrons to the side with holes than in the reverse direction.
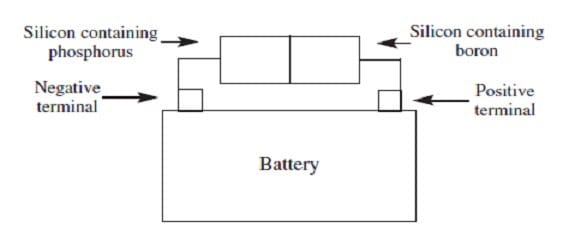
Figure 2
How does heat increase the conductivity of a semiconductor?
I) By reducing collisions between moving electrons
II) By breaking covalent bonds
III) By raising electrons to a higher energy level
A. I only
B. III only
C. I and III only
D. II and III only
-
Question 265:
In the figure below, aqueous solutions A and B are separated by a semipermeable membrane. Which of the following will occur?
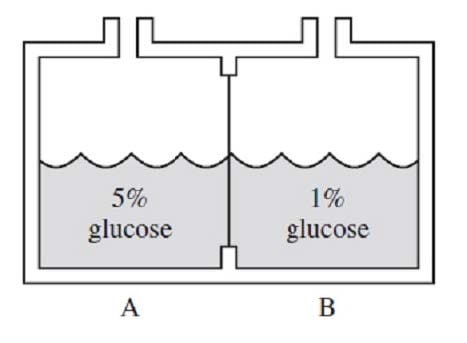
A. Glucose molecules will move from side B to side A.
B. Glucose molecules will move from side A to side B.
C. Both water and glucose molecules will move from side A to side B.
D. Water molecules will move from side B to side A.
-
Question 266:
What is the normality of a solution containing 49 g of H3PO4(MW=98 g/mol) in 2,000 mL of solution?
A. 0.25
B. 0.50
C. 0.75
D. 1.50
-
Question 267:
The mouthpiece of a telephone handset has a mass of 100 g, and the earpiece has a mass of 150 g. To balance the handset on one finger, that finger must be: (Note: Assume the bridge connecting the mouthpiece and the earpiece has a negligible mass.)
A. one and one half times farther from the earpiece than from the mouthpiece.
B. two times farther from the earpiece than from the mouthpiece
C. one and one half times farther from the mouthpiece than from the earpiece.
D. two times farther from the mouthpiece than from the earpiece
-
Question 268:
The reaction below is NOT spontaneous at any temperature.
2ICl(g) I2(g) + Cl2(g)
Which of the following is true?
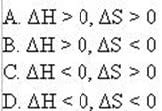
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 269:
Which of the following is the reason that water boils at a much higher temperature than does hydrogen sulfide?
A. The intramolecular O–H bonds are stronger than the intramolecular S–H bonds.
B. The enthalpy of vaporization of water is less than that of hydrogen sulfide.
C. The relative molecular mass of water is less than that of hydrogen sulfide.
D. The intermolecular O–H bonds are stronger than the intermolecular S–H bonds.
-
Question 270:
It is critical for the human body blood to maintain its pH at approximately 7.4. Decreased or increased blood pH are called acidosis and alkalosis respectively; both are serious metabolic problems that can cause death. The table below lists the major buffers found in the blood and/or kidneys. Table 1 Buffer pKa of a typical conjugate acid:*
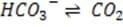
+ Histidine side chains
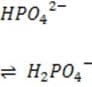
Organic phosphates N-terminal amino groups
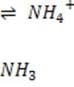
7.0
8.0
9.2
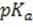
*For buffers in many of these categories, there is a range of actual values.
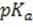
The relationship between blood pH and the of any buffer can be described by the Henderson-Hasselbalch equation:
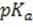
pH = + log([conjugate base]/[conjugate acid]) Equation 1
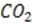
Bicarbonate, the most important buffer in the plasma, enters the blood in the form of carbon dioxide, a byproduct of metabolism, and leaves in two forms: exhaled and excreted bicarbonate. Blood pH can be adjusted rapidly by changes
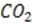
in the rate of exhalation. The reaction given below, which is catalyzed by carbonic anhydrase in the erythrocytes, describes how bicarbonate and interact in the blood.
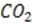
+ + Reaction 1
What would be the nature of the compensatory change that would take place in the respiratory system response to acidosis caused by organic acids?
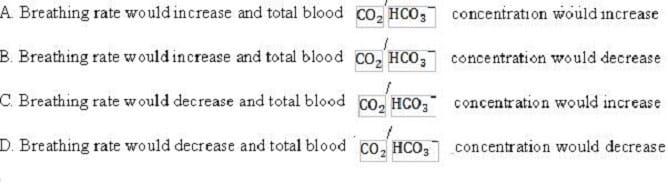
A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.