Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 251:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of
14C that was present in the organism when it died, the age of the fossil can be determined.
In determining the age of the galaxy, a technique similar to carbon dating is used on stars with the radioactive isotope 232Th, which has a half-life of 1010 years. 14C is less suitable for this application because:
A. its half-life is too long.
B. 14C is more abundant than 232Th is in stars.
C. 14C is unstable.
D. its half-life is too short.
-
Question 252:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of 14C that was present in the organism when it died, the age of the fossil can be determined. The method of carbon dating used to determine age depends upon the assumption that:
A. the half-life of 14C changes when it is ingested.
B. all ingested 14C is incorporated into the body.
C. the half-life of 14C depends on the type of molecule in which it resides.
D. the half-life of 14C does not depend upon conditions external to the 14C nucleus.
-
Question 253:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of 14C that was present in the organism when it died, the age of the fossil can be determined. The bones of a living human adult contain about 8 grams of 14C at any given time. If a prehistoric human adult skeleton is found to contain 1 gram of 14C, what is the approximate age of the fossil?
A. 5,730 years
B. 17,190 years
C. 34,380 years
D. 45,840 years
-
Question 254:
One of the most common methods that scientists use to determine the age of fossils is known as carbon dating. 14C is an unstable isotope of carbon that undergoes beta decay with a half-life of approximately 5,730 years. Beta decay occurs when a neutron in the nucleus decays to form a proton and an electron which is ejected from the nucleus. 14C is generated in the upper atmosphere when 14N, the most common isotope of nitrogen, is bombarded by neutrons. This mechanism yields a global production rate of 7.5 kg per year of 14C, which combines with oxygen in the atmosphere to produce carbon dioxide. Both the production and the decay of 14C occur simultaneously. This process continues for many half-lives of 14C, until the total amount of 14C approaches a constant. A fixed fraction of the carbon ingested by all living organisms will be 14C. Therefore, as long as an organism is alive, the ratio of 14C to 12C that it contains is constant. After the organism dies, no new 14C is ingested, and the amount of 14C contained in the organism will decrease by beta decay. The amount of 14C that must have been present in the organism when it died can be calculated from the amount of 12C present in a fossil. By comparing the amount of 14C in the fossil to the calculated amount of 14C that was present in the organism when it died, the age of the fossil can be determined.
If the global production rate of 14C were to increase to 10 kg per year:
A. the number of 14C atoms decaying per minute would increase.
B. the number of 14C atoms decaying per minute would decrease.
C. the weight of 14C on the Earth would increase indefinitely.
D. the amount of 14C ingested by living organisms would not change.
-
Question 255:
A helium-neon gas discharge laser as shown in Figure 1 below generates a coherent beam of monochromatic light at a wavelength of 632.8 nm.
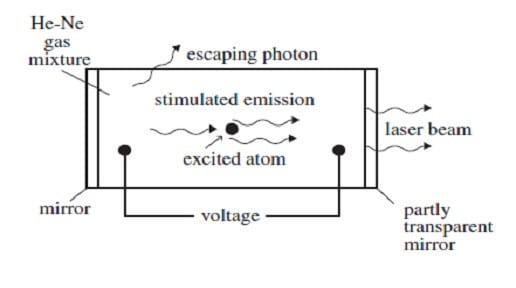
Figure 1
A discharge current of electrons is created in the tube by an applied voltage. When these electrons collide with the helium atoms, they can excite ground-state helium electrons to an energy level of 20.61 eV. The excited electrons cannot decay back to the ground state by emitting a photon because such a transition does not conserve angular momentum. Instead, if the excited helium atom collides with a neon atom, a ground-state electron in the neon atom can be excited to an energy level of 20.66 eV, and the helium electron can return to its ground state. The above process occurs quite often in the tube until the percentage of neon atoms with electrons in the 20.66-eV energy level is greater than the percentage of neon atoms with electrons in lower levels. This condition is called a population inversion. An excited electron in one of the neon atoms can then spontaneously decay by emitting a photon of wavelength 632.8 nm in a random direction. The photon will stimulate the same transition in another excited electron in a neon atom. The photon radiated by this stimulated emission process travels in the same direction as the original photon. The resulting light is then reflected back and forth inside the tube until it escapes through the partially transparent mirror. (Note: A photon's energy in eV is given by E = 1240/, where is the photon's wavelength in nm. The helium and neon ground-state energies are both 0 eV.)
Why is stimulated emission of photons necessary in order to produce a coherent beam of light instead of spontaneous emission alone?
A. Stimulated emission produces photons of higher energy than those produced by spontaneous emission.
B. Stimulated emission produces photons that travel in the same direction as the photon that induces their emission.
C. Stimulated emission produces photons with longer wavelengths than those produced by spontaneous emission.
D. Either spontaneous or stimulated emission alone would be sufficient to produce laser light.
-
Question 256:
A helium-neon gas discharge laser as shown in Figure 1 below generates a coherent beam of monochromatic light at a wavelength of 632.8 nm.
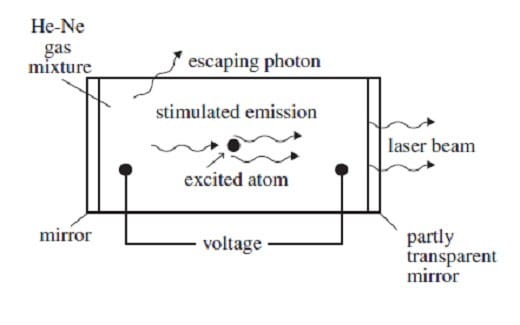
Figure 1
A discharge current of electrons is created in the tube by an applied voltage. When these electrons collide with the helium atoms, they can excite ground-state helium electrons to an energy level of 20.61 eV. The excited electrons cannot decay back to the ground state by emitting a photon because such a transition does not conserve angular momentum. Instead, if the excited helium atom collides with a neon atom, a ground-state electron in the neon atom can be excited to an energy level of 20.66 eV, and the helium electron can return to its ground state. The above process occurs quite often in the tube until the percentage of neon atoms with electrons in the 20.66-eV energy level is greater than the percentage of neon atoms with electrons in lower levels. This condition is called a population inversion. An excited electron in one of the neon atoms can then spontaneously decay by emitting a photon of wavelength 632.8 nm in a random direction. The photon will stimulate the same transition in another excited electron in a neon atom. The photon radiated by this stimulated emission process travels in the same direction as the original photon. The resulting light is then reflected back and forth inside the tube until it escapes through the partially transparent mirror. (Note: A photon's energy in eV is given by E = 1240/, where is the photon's wavelength in nm. The helium and neon ground-state energies are both 0 eV.)
A laser produces light with a wavelength of 200 nm at a power of 6.2 x 1015 eV/s. How many photons per second does this laser deliver?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 257:
A helium-neon gas discharge laser as shown in Figure 1 below generates a coherent beam of monochromatic light at a wavelength of 632.8 nm.
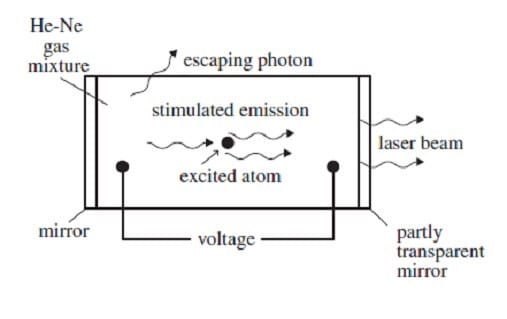
Figure 1
A discharge current of electrons is created in the tube by an applied voltage. When these electrons collide with the helium atoms, they can excite ground-state helium electrons to an energy level of 20.61 eV. The excited electrons cannot decay back to the ground state by emitting a photon because such a transition does not conserve angular momentum. Instead, if the excited helium atom collides with a neon atom, a ground-state electron in the neon atom can be excited to an energy level of 20.66 eV, and the helium electron can return to its ground state. The above process occurs quite often in the tube until the percentage of neon atoms with electrons in the 20.66-eV energy level is greater than the percentage of neon atoms with electrons in lower levels. This condition is called a population inversion. An excited electron in one of the neon atoms can then spontaneously decay by emitting a photon of wavelength 632.8 nm in a random direction. The photon will stimulate the same transition in another excited electron in a neon atom. The photon radiated by this stimulated emission process travels in the same direction as the original photon. The resulting light is then reflected back and forth inside the tube until it escapes through the partially transparent mirror. (Note: A photon's energy in eV is given by E = 1240/, where is the photon's wavelength in nm. The helium and neon ground-state energies are both 0 eV.)
A helium atom with an electron in the 20.61-eV energy level collides with a neon atom with an electron in the ground state. The result is that the helium electron returns to the ground state, and the groundstate neon electron is excited to an energy level of 20.66 eV. What is the minimum kinetic energy lost by the helium atom?
A. 0.05 eV
B. 1.96 eV
C. 10.3 eV
D. 20.61 eV
-
Question 258:
A helium-neon gas discharge laser as shown in Figure 1 below generates a coherent beam of monochromatic light at a wavelength of 632.8 nm.
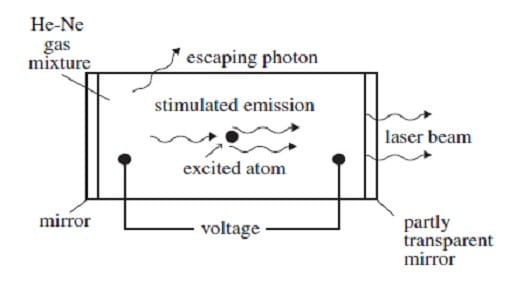
Figure 1
A discharge current of electrons is created in the tube by an applied voltage. When these electrons collide with the helium atoms, they can excite ground-state helium electrons to an energy level of 20.61 eV. The excited electrons cannot decay back to the ground state by emitting a photon because such a transition does not conserve angular momentum. Instead, if the excited helium atom collides with a neon atom, a ground-state electron in the neon atom can be excited to an energy level of 20.66 eV, and the helium electron can return to its ground state. The above process occurs quite often in the tube until the percentage of neon atoms with electrons in the 20.66-eV energy level is greater than the percentage of neon atoms with electrons in lower levels. This condition is called a population inversion. An excited electron in one of the neon atoms can then spontaneously decay by emitting a photon of wavelength 632.8 nm in a random direction. The photon will stimulate the same transition in another excited electron in a neon atom. The photon radiated by this stimulated emission process travels in the same direction as the original photon. The resulting light is then reflected back and forth inside the tube until it escapes through the partially transparent mirror. (Note: A photon's energy in eV is given by E = 1240/, where is the photon's wavelength in nm. The helium and neon ground-state energies are both 0 eV.)
What is the energy of the photon with wavelength 632.8 nm?
A. 0.05 eV
B. 1.96 eV
C. 20.61 eV
D. 20.66 eV
-
Question 259:
A helium-neon gas discharge laser as shown in Figure 1 below generates a coherent beam of monochromatic light at a wavelength of 632.8 nm.
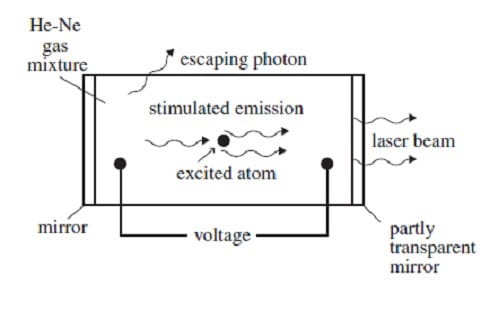
Figure 1
A discharge current of electrons is created in the tube by an applied voltage. When these electrons collide with the helium atoms, they can excite ground-state helium electrons to an energy level of 20.61 eV. The excited electrons cannot decay back to the ground state by emitting a photon because such a transition does not conserve angular momentum. Instead, if the excited helium atom collides with a neon atom, a ground-state electron in the neon atom can be excited to an energy level of 20.66 eV, and the helium electron can return to its ground state. The above process occurs quite often in the tube until the percentage of neon atoms with electrons in the 20.66-eV energy level is greater than the percentage of neon atoms with electrons in lower levels. This condition is called a population inversion. An excited electron in one of the neon atoms can then spontaneously decay by emitting a photon of wavelength 632.8 nm in a random direction. The photon will stimulate the same transition in another excited electron in a neon atom. The photon radiated by this stimulated emission process travels in the same direction as the original photon. The resulting light is then reflected back and forth inside the tube until it escapes through the partially transparent mirror. (Note: A photon's energy in eV is given by E = 1240/, where is the photon's wavelength in nm. The helium and neon ground-state energies are both 0 eV.)
A population inversion exists in the laser tube when:
A. the percentage of neon atoms with electrons in the ground state is greater than the percentage of neon atoms with electrons in higher energy levels.
B. the percentage of neon atoms with electrons in a higher energy level is greater than the percentage of neon atoms with electrons in lower energy levels.
C. the percentage of neon atoms with electrons in a higher energy level is equal to the percentage of neon atoms with electrons in the ground state.
D. all the neon atoms have electrons in the ground state only.
-
Question 260:
Band theory explains the conductivity of certain solids by stating that the atomic orbitals of the individual atoms in the solid merge to produce a series of atomic orbitals comprising the entire solid. The closely-spaced energy levels of the orbitals form bands. The band corresponding to the outermost occupied subshell of the original atoms is called the valence band. If partially full, as in metals, it serves as a conduction band through which electrons can move freely. If the valence band is full, then electrons must be raised to a higher band for conduction to occur. The greater the band gap between the separate valence and conduction bands, the poorer the material's conductivity. Figure 1 shows the valence and conduction bands of a semiconductor, which is intermediate in conductivity between conductors and insulators.
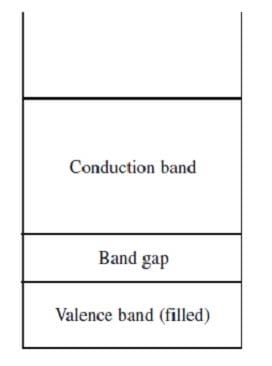
Figure 1
When silicon, a semiconductor with tetrahedral covalent bonds, is heated, a few electrons escape into the conduction band. Doping the silicon with a few phosphorus atoms provides unbonded electrons that escape more easily, increasing conductivity. Doping with boron produces holes in the bonding structure, which may be filled by movement of nearby electrons within the lattice. When a semiconductor in an electric circuit has excess electrons on one side and holes on the other, electron flow occurs more easily from the side with excess electrons to the side with holes than in the reverse direction.
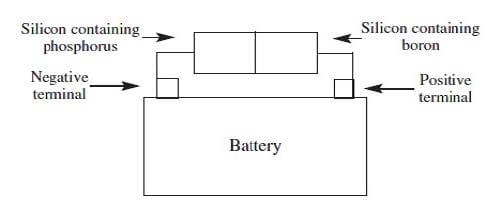
Figure 2 If the semiconductor orientation in Figure 2 were reversed so that the boron-doped silicon was on the left and the phosphorus-doped silicon on the right, what could be said about the electron flow of the new setup?
A. The electron flow is easier in the new direction than in that of Figure 2.
B. The electron flow is the same in either direction.
C. The electron flow is more difficult in the new direction than in that of Figure 2.
D. The electrons cannot flow in the new setup.
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.