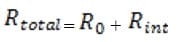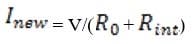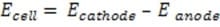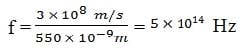Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 281:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode "sandwiched" between 2 lead anodes. Insulating separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
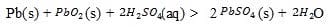
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E?V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2ePbSO4(s) + 2H2O
PbSO4(s) + 2e-Pb(s) + SO42-(aq)
1.69
?.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery is said to be "dead" when Reaction 1 has proceeded completely to the right.
Often in cold weather the battery goes "dead". Thermodynamic data confirms that the voltage of most electrochemical cells decreases with decreasing temperature. If the battery is warmed to room temperature, it often recovers its ability to deliver normal power. The battery appeared "dead" because:
I) the resistance of the electrolyte had decreased. II) the viscosity of the electrolyte had increased. III) the viscosity of the electrolyte had decreased.
A. I only
B. II only
C. I and II only
D. I and III only
-
Question 282:
The resistance of a resistor is defined as the ratio of the voltage drop across it to the current passing through it. The resistance of a resistor can be measured using the circuit illustrated in Figure 1.
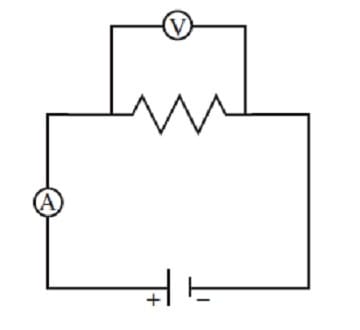
Figure 1
In the above circuit, a variable voltage source with negligible internal resistance is connected to a resistor. The voltage across the resistor is measured by a voltmeter and the current through the resistor is measured by an ammeter.
Additional resistors may be added to the circuit. The total resistance can be calculated as follows: If and are two resistances of two resistors, then the total resistance is given by = + when the resistors are connected in

series, and by 1/ = 1/ + 1/ when the resistors are connected in parallel.
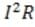
Circuits similar to the one above are used in the common household appliance known as the toaster. The rate by which energy in the form of heat is dissipated by the resistor equals , where I is the current that passes through the resistor and R is the resistance of the resistor. Energy is dissipated in a resistor because moving electrons collide with atoms in the resistor, causing the atoms to vibrate.
The variable voltage source in the circuit in Figure 1 is replaced by a battery connected in series with the resistor and ammeter. The battery has a small internal resistance. How will the circuit be affected?
A. The current measured by the ammeter at a specific voltage will be greater in the circuit with the battery than in the old circuit.
B. The current measured by the ammeter at a specific voltage will be smaller in the circuit with the battery than in the old circuit.
C. The resistance of the resistor at a specific voltage will be greater in the circuit with the battery than in the old circuit.
D. The resistance of the resistor at a specific voltage will be smaller in the circuit with the battery than in the old circuit.
-
Question 283:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode "sandwiched" between 2 lead anodes. Insulating separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
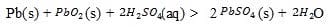
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E?V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2ePbSO4(s) + 2H2O
PbSO4(s) + 2e-Pb(s) + SO42-(aq)
1.69
?.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery
is said to be "dead" when Reaction 1 has proceeded completely to the right.
The graph below shows the change in potential versus time of a 12-V lead storage battery during discharge.
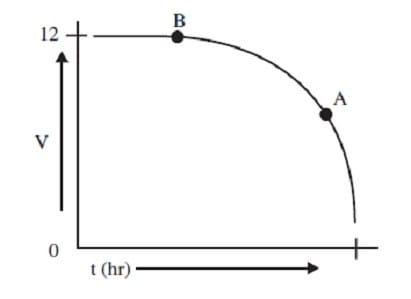
Which of the following is true?
A. The electrolyte density at point A is greater than it is at point B.
B. The electrolyte density at point A is less than it is at point B.
C. The electrolyte density at point A is the same as it is at point B.
D. The electrolyte density at points A and B cannot be compared without more information.
-
Question 284:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode "sandwiched" between 2 lead anodes. Insulating separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
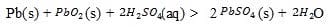
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E?V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2ePbSO4(s) + 2H2O
PbSO4(s) + 2e-Pb(s) + SO42-(aq)
?.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery
is said to be "dead" when Reaction 1 has proceeded completely to the right.
Where does oxidation occur in the lead storage battery?
A. At the lead oxide cathodes
B. At the lead oxide anodes
C. At the lead cathodes
D. At the lead anodes
-
Question 285:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode "sandwiched" between 2 lead anodes. Insulating separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
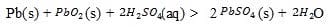
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E?V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2ePbSO4(s) + 2H2O
PbSO4(s) + 2e-Pb(s) + SO42-(aq)
?.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery
is said to be "dead" when Reaction 1 has proceeded completely to the right.
Which of the following occurs as the battery is being recharged?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 286:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode “sandwiched” between 2 lead anodes. Insulating
separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E°(V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2e-→
PbSO4(s) + 2H2O
PbSO4(s) + 2e-→ Pb(s) + SO42-(aq)
–0.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery
is said to be “dead” when Reaction 1 has proceeded completely to the right.
How many cells would be required to produce a 20-volt lead-acid battery of the type described in the passage?
A. 5
B. 10
C. 15
D. 20
-
Question 287:
The lead-acid battery, also called a lead storage battery, is the battery of choice for starting automobiles. It contains 6 cells connected in series, each composed of a lead oxide cathode "sandwiched" between 2 lead anodes. Insulating separators are placed between the electrodes to prevent internal short-circuits. Aqueous sulfuric acid is the electrolyte.
When the battery is being discharged, the following reaction takes place:
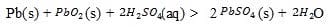
Reaction 1
The electrode reactions, both written as reductions, are shown in Table 1.
Table 1
Half-reaction
E?V)
PbO2(s) + SO42-(aq) + 4H+(aq) + 2ePbSO4(s) + 2H2O
PbSO4(s) + 2e-Pb(s) + SO42-(aq)
1.69
?.36
As a car operates, the battery is recharged by electricity produced by the car's alternator, an AC generator whose ultimate power source is the car's internal combustion engine. In spite of this, batteries eventually lose their power. The battery is said to be "dead" when Reaction 1 has proceeded completely to the right.
Which reaction takes place at the anode as the battery is discharging?
A. The first half-reaction, proceeding to the left
B. The first half-reaction, proceeding to the right
C. The second half-reaction, proceeding to the left
D. The second half-reaction, proceeding to the right
-
Question 288:
A continuous spectrum of light, sometimes called blackbody radiation, is emitted from a region of the Sun called the photosphere. Although the continuous spectrum contains light of all wavelengths, the intensity of the emitted light is much
greater at some wavelengths than at others. The relationship between the most intense wavelength of blackbody radiation and the temperature of the emitting body is given by Wien's law, λ = 2.9 x 106 /T, where λ is the wavelength in
nanometers and T is the temperature in kelvins.
As the blackbody radiation from the Sun passes through the cooler gases in the Sun's atmosphere, some of the photons are absorbed by the atoms in these gases. A photon will be absorbed if it has just enough energy to excite an electron
from a lower energy state to a higher one. The absorbed photon will have an energy equal to the energy difference between these two states. The energy of a photon is given by E = hf = hc/λ where h = 6.63 × 10-34 J•s, Planck's constant,
and c = 3 × 108 m/s, the speed of light in a vacuum.
The Sun is composed primarily of hydrogen. Electron transitions in the hydrogen atom from energy state n = 2 to higher energy states are listed below along with the energy of the absorbed photon:
Final Energy State
Energy (x 10-19 J)
n = 3
3.02
n = 4
4.08
n = 5
4.57 n = 6
4.84
n = ∞
5.44
At the center of the visible spectrum is light with a wavelength of 550 nm. What is the frequency of this light?
A. 9.0 x 108 Hz
B. 1.8 x 1012 Hz
C. 5.4 x 1014 Hz
D. 1.8 x 1016 Hz
-
Question 289:
A continuous spectrum of light, sometimes called blackbody radiation, is emitted from a region of the Sun called the photosphere. Although the continuous spectrum contains light of all wavelengths, the intensity of the emitted light is much greater at some wavelengths than at others. The relationship between the most intense wavelength of blackbody radiation and the temperature of the emitting body is given by Wien's law, = 2.9 x 106 /T, where is the wavelength in nanometers and T is the temperature in kelvins.
As the blackbody radiation from the Sun passes through the cooler gases in the Sun's atmosphere, some of the photons are absorbed by the atoms in these gases. A photon will be absorbed if it has just enough energy to excite an electron from a lower energy state to a higher one. The absorbed photon will have an energy equal to the energy difference between these two states. The energy of a photon is given by E = hf = hc/ where h = 6.63 x 10-34 Js, Planck's constant, and c = 3 x 108 m/s, the speed of light in a vacuum.
The Sun is composed primarily of hydrogen. Electron transitions in the hydrogen atom from energy state n = 2 to higher energy states are listed below along with the energy of the absorbed photon:
Final Energy State Energy (x 10-19 J) n = 3
3.02
n = 4
4.08
n = 5
4.57 n = 6
4.84 n =
5.44
If a star suddenly doubles in size but remains at the same temperature, how does its continuous spectrum change?
A. The peak intensity occurs at the same wave-length.
B. The peak intensity occurs at a longer wave-length.
C. The peak intensity occurs at a shorter wave-length.
D. The intensity peak narrows.
-
Question 290:
A continuous spectrum of light, sometimes called blackbody radiation, is emitted from a region of the Sun called the photosphere. Although the continuous spectrum contains light of all wavelengths, the intensity of the emitted light is much greater at some wavelengths than at others. The relationship between the most intense wavelength of blackbody radiation and the temperature of the emitting body is given by Wien's law, λ = 2.9 x 106 /T, where λ is the wavelength in nanometers and T is the temperature in kelvins.
As the blackbody radiation from the Sun passes through the cooler gases in the Sun's atmosphere, some of the photons are absorbed by the atoms in these gases. A photon will be absorbed if it has just enough energy to excite an electron from a lower energy state to a higher one. The absorbed photon will have an energy equal to the energy difference between these two states. The energy of a photon is given by E = hf = hc/λ where h = 6.63 x 10-34 J•s, Planck's constant, and c = 3 x 108 m/s, the speed of light in a vacuum.
The Sun is composed primarily of hydrogen. Electron transitions in the hydrogen atom from energy state n = 2 to higher energy states are listed below along with the energy of the absorbed photon:
Final Energy State Energy (x 10-19 J) n = 3
3.02
n = 4
4.08
n = 5
4.57 n = 6
4.84
n = ∞
5.44
If the temperature of the Sun's photosphere is 5800 K, what wavelength of radiation does the Sun emit with the greatest intensity?
A. 2 nm
B. 50 nm
C. 500 nm
D. 4,500 nm
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.