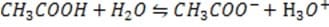Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 301:
The simple harmonic motion of a mass suspended from vertical springs is investigated in two experiments. The springs used in both experiments have a spring constant k and a natural length L0. The material used to make the springs has a
Young's modulus of 2 x 1011 Pa.
In the first experiment a mass m is suspended from a spring. The mass stretches the spring to a new length L, called the equilibrium length.
In the second experiment the mass m is suspended from two identical springs as shown in Figure 2 below. When the mass m is in equilibrium, each spring is stretched from its natural length by the same amount xe.
In both experiments the masses of the springs are negligible, and the elastic limits of the springs are never exceeded.
The mass in the first experiment is pulled down a distance A from its equilibrium position and then released from rest. The mass will then oscillate with simple harmonic motion. As the mass moves up and down, energy is dissipated due to factors such as air resistance and internal heating of the spring. The mass will no longer oscillate when the total energy dissipated equals:
A. kL2/2
B. kA2/2
C. k(L + A)2/2
D. kL0 2/2
-
Question 302:
The simple harmonic motion of a mass suspended from vertical springs is investigated in two experiments. The springs used in both experiments have a spring constant k and a natural length L0. The material used to make the springs has a
Young's modulus of 2 x 1011 Pa.
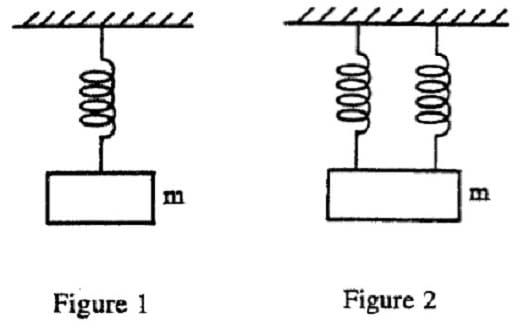
In the first experiment a mass m is suspended from a spring. The mass stretches the spring to a new length L, called the equilibrium length.
In the second experiment the mass m is suspended from two identical springs as shown in Figure 2 below. When the mass m is in equilibrium, each spring is stretched from its natural length by the same amount xe.
In both experiments the masses of the springs are negligible, and the elastic limits of the springs are never exceeded.
In the first experiment the mass is pulled down and set into motion. The position of greatest speed is:
A. at the equilibrium position.
B. at the position where the spring's length is its natural length.
C. at the lowest point in its motion.
D. at the highest point in its motion.
-
Question 303:
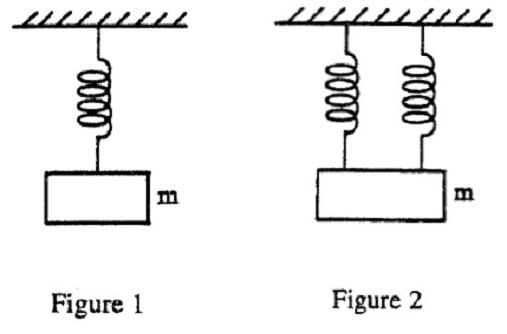
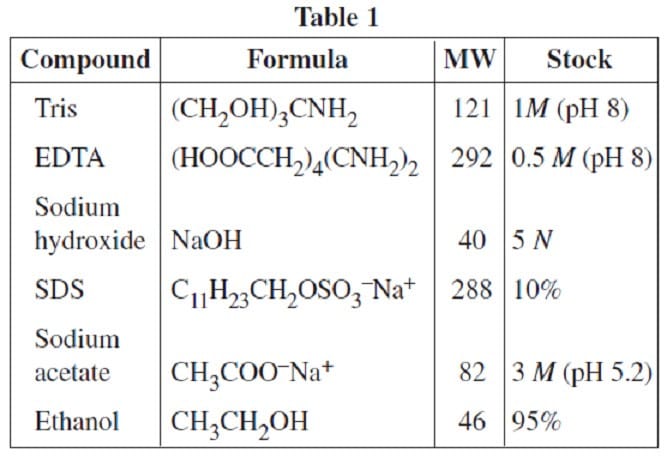
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at –20°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.
What would be the pH of 100 mL of the sodium acetate stock solution after the addition of 3.6 g of HCl? (pKa of acetic acid = 4.74)
A. 1.0
B. 4.74
C. 5.2
D. 6.0
-
Question 304:
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at –20°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.

Which of the following conclusions can be reached based on the fact that DNA precipitates in the last step of the plasmid prep procedure?
A. DNA dissolves better in water at lower temperatures.
B. DNA is polar and therefore dissolves better in water than in a mixture of water and ethanol.
C. DNA is nonpolar and therefore dissolves better in ethanol than in water.
D. DNA dissolves well in ethanol and precipitates only because the solution is centrifuged.
-
Question 305:
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at –20°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.

Pure ethanol (CH3CH2OH) is difficult to prepare and therefore expensive; 95% ethanol is much cheaper. Consequently, 95% ethanol is generally used in the preparation of dilute ethanol solutions. How much 95% ethanol would be needed to produce a 500 mL solution of 70% ethanol by volume in water?
A. 333 mL
B. 350 mL
C. 368 mL
D. 475 mL
-
Question 306:
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at –20°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.

What is the molality of a stock solution that is 10% SDS by mass?
A. 0.028 m
B. 0.100 m
C. 0.347 m
D. 0.385 m
-
Question 307:
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at ?0°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.
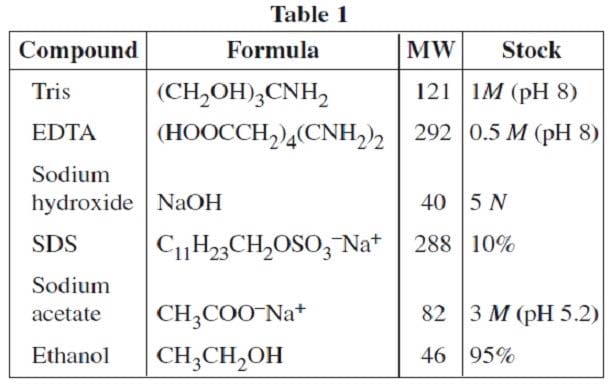
EDTA is available commercially in the form of a hydrated sodium salt, Na2 EDTA ?2H2O. How much of this salt must be used to produce 1 L of a 0.5 M stock solution?
A. 145 g
B. 146 g
C. 186 g
D. 187 g
-
Question 308:
A researcher in a molecular biology lab planned to carry out an extraction procedure known as an alkaline plasmid prep, which is designed to purify plasmids, small pieces of the hereditary material DNA, from bacterial cells. The bacteria are first placed into a test tube containing liquid nutrient medium and allowed to grow until they reach a high population density. The culture, which consists of solid cells suspended in the medium, is then centrifuged; a solid pellet is formed. The supernatant is poured out, leaving the pellet behind, and the cells are resuspended in a mL of lysis buffer solution (50 mM glucose, 25 mM Tris buffer and 10 mM ethylenediaminetetraacetic acid (EDTA), with 5 mg of the enzyme lysozyme added). They are then incubated for 30 minutes at 0°C, during which time the bacterial cell walls break down and the cell contents are released into the solution. After incubation, 1 mL of 0.4 N sodium hydroxide and 1 mL of 2% sodium dodecyl sulfate (SDS) are added, and the solution is again incubated on ice for 10 minutes. 2 mL of 3 M sodium acetate are added and the mixture is incubated for 30 minutes at 0°C. The test tube is centrifuged once more and the supernatant is decanted into a clean tube, leaving behind the protein and most other cell components in the pellet. Finally, 10 mL of pure ethanol are added to the supernatant from the previous step to precipitate out the DNA, and the test tube is incubated at ?0°C for 60 minutes, during which the mixture remains liquid. The mixture is centrifuged a final time and the supernatant removed. The translucent precipitate that results is washed with 70% ethanol (70% ethanol and 30% water by volume), allowed to dry, and resuspended in 1 mL of TE buffer (10 mM Tris, 1 mM EDTA). In preparation for this experiment, the researcher prepared stock solutions of the various chemicals that she will need in the experiment. Stock solutions are highly concentrated solutions of commonly used chemicals in water from which dilute solutions are prepared for daily use. Table 1 shows the chemicals, their molecular formulas and weights, and the composition of commonly used stock solutions.

Tris (Tris(hydroxymethyl)aminomethane) is generally used as a buffer. If pH 8.0 is a good buffering region for Tris, then:
I) the pKa of Tris must be near pH 8.0
II) if Tris is titrated with acid, the titration curve will possess a steep region near pH 8.0.
III) a great deal of NaOH would have to be added to pH 8.0 Tris in order to significantly affect the pH.
A. I only
B. III only
C. I and II only
D. I and III only
-
Question 309:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are high-energy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.
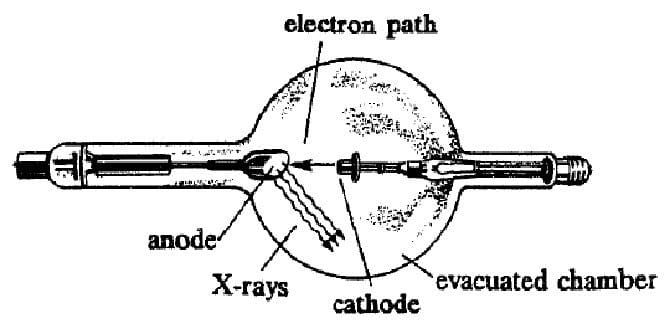
There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
In an X-ray tube, electrons of charge e are accelerated through a potential difference of V. The anode is cooled by water of mass m with specific heat c. If n electrons per second strike the anode, what is the maximum possible rise in the temperature of the water after 100 s?
A. nVe/100mc
B. 100Ve/mc
C. 100n(Ve + mc)
D. 100nVe/mc
-
Question 310:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are highenergy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.

There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
Which of the following graphs best represents the relationship between the amount of X-rays absorbed per unit length of material and the energy of the X-rays, for lead, bone, and air?

A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.