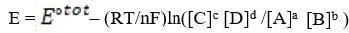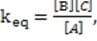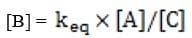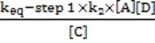Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 311:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are highenergy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.
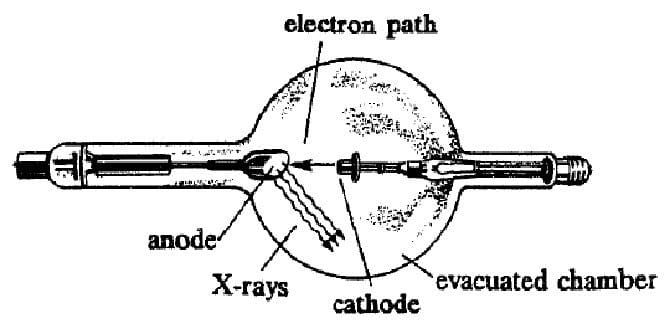
There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
What is the minimum potential difference required to produce a 0.06 nm X-ray from an electron transition in a metal?
A. 15,000 V
B. 20,000 V
C. 20,500 V
D. 21,500 V
-
Question 312:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are high-energy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.

There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
An X-ray source produces X-rays with a maximum frequency of 6 x 108 Hz. If the cathode current is doubled so that twice as many electrons are emitted per unit time, what is the new maximum frequency of the X-rays produced?
A. 3 x 1018 Hz
B. 6 x 1018 Hz
C. 12 x 1018 Hz
D. 24 x 1018 Hz
-
Question 313:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are high-energy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.

There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
What is the direction of the electric field that accelerates the electrons?
A. From the anode toward the cathode
B. From the cathode toward the anode
C. Into the page
D. Out of the page
-
Question 314:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are high energy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.

There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10–15 eV•s; speed of light c = 3 x 108 m/s.)
How does the wavelength of an X-ray produced from a K-alpha transition in molybdenum compare to that produced from a lower energy K-alpha transition in copper?
A. It is shorter.
B. It is the same.
C. It is longer.
D. It depends on the energy of the incoming electron.
-
Question 315:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
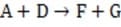
Step 1
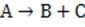
(Slow) Step 2
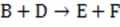
(fast) Step 3
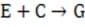
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
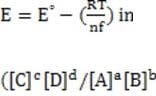
Equation 1 When
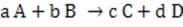
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 x 104 C/mol.)
Catalysts are effective in increasing the rate of a reaction because they:
A. increase the energy of the activated complex.
B. increase the value of the equilibrium constant.
C. decrease the number of collisions between reactant molecules.
D. lower the activation energy
-
Question 316:
X-rays are produced by a device which beams electrons with an energy between 103 and 106 eV at a metal plate. The electrons interact with the metal plate and are stopped by it. Much of the energy of the incoming electrons is released in the form of X-rays, which are high-energy photons of electromagnetic radiation. An example of such a device is shown below. Electrons are accelerated from the cathode towards the anode by an electric field.

There are two mechanisms by which the X-rays are produced within the metal. The first mechanism is called bremsstrahlung, which is German for "breaking radiation." X-rays are emitted by the electrons as they are brought to rest by
interactions with the positive nuclei of the anode.
The second mechanism occurs when an incoming electron knocks an inner electron out of one of the metal atoms of the anode. This electron is replaced by an electron from a higher energy level of the atom, and a photon making up the
energy difference is emitted.
X-rays are absorbed by a material when they pass through it. The amount of X-rays absorbed increases with the density of the material. In addition, lower energy X-rays are more likely to be absorbed than higher energy X-rays. (Note: 1 eV =
1.6 x 1019 J; Planck's constant h = 4.1 x 10-15 eV•s; speed of light c = 3 x 108 m/s.)
An electron is accelerated through a distance of 0.1 m by a potential difference of 10,000 volts. What is the electron's energy as it strikes the anode?
A. 100 eV
B. 1,000 eV
C. 10,000 eV
D. 1 J
-
Question 317:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
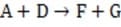
Step 1
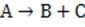
(Slow) Step 2
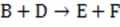
(fast) Step 3
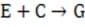
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
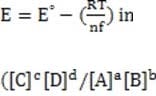
Equation 1 When
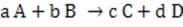
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 104 C/mol.)
What would be the cell emf of the following system at 298K?
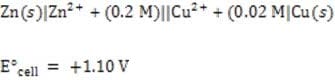
A. 1.07 V
B. 1.10 V
C. 1.13 V
D. 1.20 V
-
Question 318:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
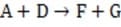
Step 1
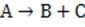
(Slow) Step 2
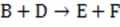
(fast) Step 3
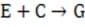
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx, where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:

Equation 1 When
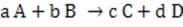
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 x 104 /mol.)
What is the effect of increasing the concentration of reactants in a voltaic cell?
A. The voltage increases, while the spontaneity of the reaction remains the same.
B. The spontaneity of the reaction increases, but the voltage remains the same.
C. Both the voltage and the spontaneity of the reaction increase.
D. The reaction rate increases, but the voltage and spontaneity of the reaction are unchanged.
-
Question 319:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
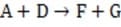
Step 1
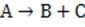
(Slow) Step 2
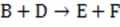
(fast) Step 3
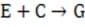
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
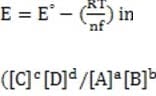
Equation 1 When
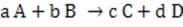
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 x 104 C/mol.)
Which of the following is true of a reaction at equilibrium?
I) k1/k-1 = 1
II) E = E°
III) ln([C]c [D]d /[A]a [B]b ) = nFE°/RT
A. I only
B. III only
C. I and II only
D. I, II, and III
-
Question 320:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
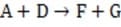
Step 1
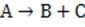
(Slow) Step 2
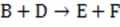
(fast) Step 3
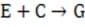
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
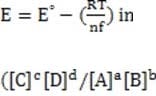
Equation 1 When
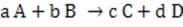
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 x 104 C/mol.)
If Step 2 above were the rate-determining step of Reaction 1, which of the following equations would correctly define the rate?

A. Option A
B. Option B
C. Option C
D. Option D
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.