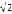Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 321:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
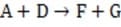
Step 1
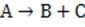
(Slow) Step 2
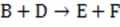
(fast) Step 3
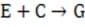
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
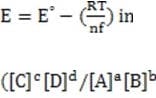
Equation 1 When
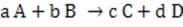
Reaction 2
Note: R = 8.314 J/K•mol; F = 9.6485 x 104 C/mol.)
In a test of the rate of Step 3 of Reaction 1, a solution is prepared containing a 0.1 M concentration of E and a 50 M concentration of C. The rate is calculated after the reaction has gone 50% to completion. By what percent will the calculated
rate differ from the true rate if we treat the reaction as pseudo first-order?
A. 0.02%
B. 0.05%
C. 0.1%
D. 0.2%
-
Question 322:
A gibbon (lesser ape) of mass m and arm length l reaches to a branch level with its shoulder and starts to swing with its arm fully extended. At the bottom of the swing, its velocity is: A. Option A

B. Option B
C. Option C
D. Option D
-
Question 323:
Several techniques have been developed to determine the order of a reaction. The rate of a reaction cannot be predicted on the basis of the overall equation, but can be predicted on the basis of the rate-determining step. For instance, the following reaction can be broken down into three steps.
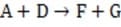
Step 1
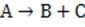
(Slow) Step 2
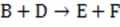
(fast) Step 3
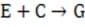
(fast)
Reaction 1 In this case, the first step in the reaction pathway is the rate-determining step. Therefore, the overall rate of the reaction must equal the rate of the first step, k1 [A] where k1 is the rate constant for the first step. (Rate constants of the different steps are denoted by kx , where x is the step number.)
In some cases, it is desirable to measure the rate of a reaction in relation to only one species. In a second-order reaction, for instance, a large excess of one species is included in the reaction vessel. Since a relatively small amount of this large concentration is reacted, we assume that the concentration essentially remains unchanged. Such a reaction is called a pseudo first-order reaction. A new rate constant, k', is established, equal to the product of the rate constant of the original reaction, k, and the concentration of the species in excess. This approach is often used to analyze enzyme activity.
In some cases, the reaction rate may be dependent on the concentration of a short-lived intermediate. This can happen if the rate-determining step is not the first step. In this case, the concentration of the intermediate must be derived from the equilibrium constant of the preceding step. For redox reactions, the equilibrium can be correlated with the voltage produced by two half-cells by means of the Nernst equation. This equation states that at any given moment:
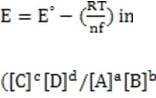
Equation 1 When
Reaction 2
Note: R = 8.314 J/K?mol; F = 9.6485 x 104 C/mol.)
An enzyme, R, catalyzes the oxidation of A to B. Reacting various concentrations of A and B with a large excess of R produced the following results during the first few minutes of the reaction.
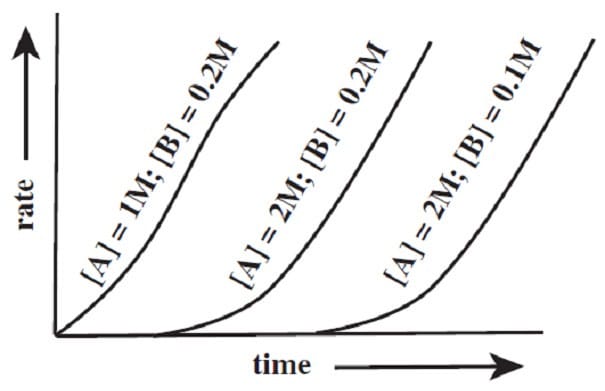
Which of the following is the best tentative rate equation?
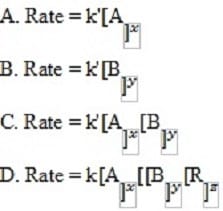
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 324:
Which titration curve would be produced by titrating 25 mL of a 0.1 N weak base with a 0.1 N strong acid?
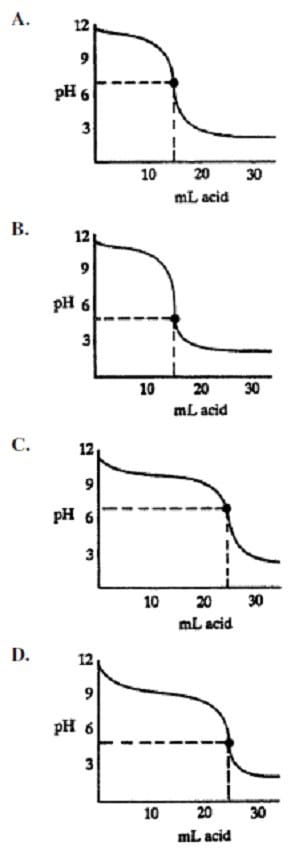
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 325:
Suppose an -particle starting from rest is accelerated through a 5 megavolt potential difference. What is the final kinetic energy of the -particle? (Note: Assume that e = 1.6x10-19 C.)
A. 1.6 x 10-12 J
B. 8.0 x 10-13 J
C. 6.4 x 10-26 J
D. 3.2 x 10-26 J
-
Question 326:
Based on the table below, what is the cell voltage for the following reaction?
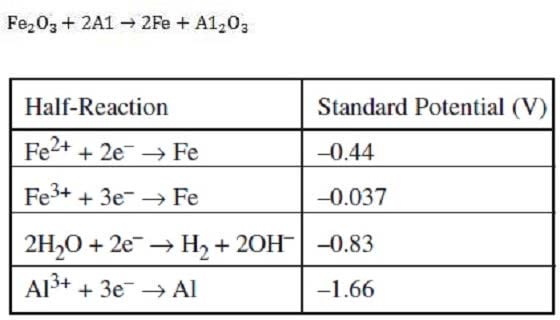
A. –1.33 V
B. 1.99 V
C. 1.33 V
D. 1.62 V
-
Question 327:
A particle of mass m moves in a circle of radius r at a uniform speed and makes 1 revolution per second. What is the energy of the particle?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 328:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30° to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45° to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.

Figure 1
The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp,
thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
What is the acceleration of an 80-kg skier going down the track if = 45?

A. Option A
B. Option B
C. Option C
D. Option D
-
Question 329:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30° to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45° to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.

Figure 1
The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp, thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
If a skier uses skis of greater surface area, which of the following would occur?
A. The normal force of the slope on the skier would increase.
B. The normal force of the slope on the skier would decrease.
C. The pressure exerted on the slope by the skis would increase.
D. The pressure exerted on the slope by the skis would decrease.
-
Question 330:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30?to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45?to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.
Figure 1

The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp, thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
Which of the following would increase the jump distance?
I) Increasing the vertical height h of the jump track II) Increasing the angle of incline of the jump track III) Carrying extra weight to increase the total mass of the ski jumper
A. I only
B. I and II only
C. II and III only
D. I and III only
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.