Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 481:
Before birth, the rodent brain is sexually undifferentiated. It is only in the first few days following birth, during a period referred to as the critical period, that the rodent brain differentiates along male or female lines. The hormone testosterone plays a critical role in this development. Specifically, sexual differentiation is determined by the presence of estradiol, an estrogen derivative of testosterone, in certain areas of the brain. Testosterone is converted to estradiol in critical brain cells that contain the enzyme aromatase. To study the effects of testosterone on the neonatal rodent brain, the following experiments were conducted: The above research, combined with additional studies, concluded that testosterone has two "organizational" effects on the male rodent brain: Defeminization Moderate levels of testosterone-derived estradiol during the critical period are sufficient for defeminization of the brain. Defeminization of the rodent brain results in loss of estrogen positive feedback on LH and FSH secretion and the ensuing loss of cyclicity, as well as loss of female sex behavior. Masculinization High levels of estradiol due to high levels of testosterone during the critical period results in masculinization of the brain. Masculinization leads to the induction of male sex behavior including antagonism towards other males and the mounting of females.
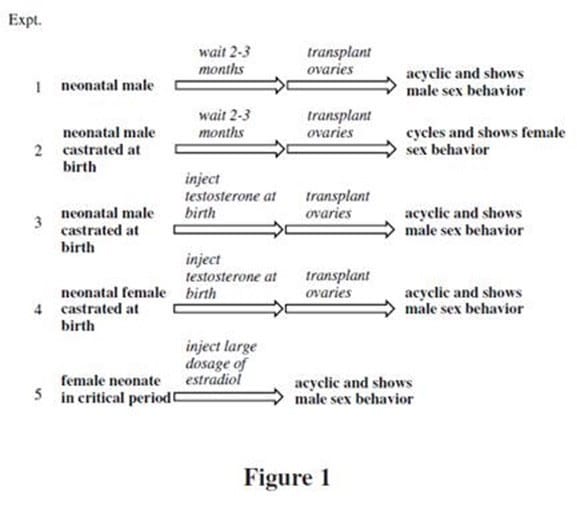
In Experiment 2, researchers most likely waited 2-3 months before implanting ovaries:
A. to allow the rat to recover from previous surgery.
B. to allow testosterone levels to rise to necessary concentrations.
C. to wait for the critical period to pass.
D. to promote the defeminization of the rat brain.
-
Question 482:
Before birth, the rodent brain is sexually undifferentiated. It is only in the first few days following birth, during a period referred to as the critical period, that the rodent brain differentiates along male or female lines. The hormone testosterone plays a critical role in this development. Specifically, sexual differentiation is determined by the presence of estradiol, an estrogen derivative of testosterone, in certain areas of the brain. Testosterone is converted to estradiol in critical brain cells that contain the enzyme aromatase. To study the effects of testosterone on the neonatal rodent brain, the following experiments were conducted:

The above research, combined with additional studies, concluded that testosterone has two "organizational" effects on the male rodent brain: Defeminization Moderate levels of testosterone-derived estradiol during the critical period are sufficient for defeminization of the brain. Defeminization of the rodent brain results in loss of estrogen positive feedback on LH and FSH secretion and the ensuing loss of cyclicity, as well as loss of female sex behavior. Masculinization High levels of estradiol due to high levels of testosterone during the critical period results in masculinization of the brain. Masculinization leads to the induction of male sex behavior including antagonism towards other males and the mounting of females.
A researcher proposes that very low doses of estradiol are required for induction of female sex behavior and cyclicity in the rodent brain. Which of the following observations would best support this hypothesis?
A. Female neonates injected with the anti-estrogen tamoxifen are acyclic and show neither male nor female sex behavior.
B. Female neonates lacking the aromatase enzyme develop normally.
C. Female neonates injected with large doses of estradiol are acyclic and show male sex behavior.
D. Male neonates injected with low dosages of estradiol develop normally.
-
Question 483:
Before birth, the rodent brain is sexually undifferentiated. It is only in the first few days following birth, during a period referred to as the critical period, that the rodent brain differentiates along male or female lines. The hormone testosterone plays a critical role in this development. Specifically, sexual differentiation is determined by the presence of estradiol, an estrogen derivative of testosterone, in certain areas of the brain. Testosterone is converted to estradiol in critical brain cells that contain the enzyme aromatase. To study the effects of testosterone on the neonatal rodent brain, the following experiments were conducted:

The above research, combined with additional studies, concluded that testosterone has two "organizational" effects on the male rodent brain: Defeminization Moderate levels of testosterone-derived estradiol during the critical period are sufficient for defeminization of the brain. Defeminization of the rodent brain results in loss of estrogen positive feedback on LH and FSH secretion and the ensuing loss of cyclicity, as well as loss of female sex behavior. Masculinization High levels of estradiol due to high levels of testosterone during the critical period results in masculinization of the brain. Masculinization leads to the induction of male sex behavior including antagonism towards other males and the mounting of females.
A mutant male neonatal rodent with a defective aromatase enzyme is injected with large doses of testosterone during the critical period. Which of the following would occur?
A. Induction of male sex behavior
B. Development of female sex behavior
C. Loss of both cyclicity and female sex behavior
D. Loss of both cyclicity and female sex behavior and induction of male sex behavior
-
Question 484:
Before birth, the rodent brain is sexually undifferentiated. It is only in the first few days following birth, during a period referred to as the critical period, that the rodent brain differentiates along male or female lines. The hormone testosterone plays a critical role in this development. Specifically, sexual differentiation is determined by the presence of estradiol, an estrogen derivative of testosterone, in certain areas of the brain. Testosterone is converted to estradiol in critical brain cells that contain the enzyme aromatase. To study the effects of testosterone on the neonatal rodent brain, the following experiments were conducted:

The above research, combined with additional studies, concluded that testosterone has two "organizational" effects on the male rodent brain: Defeminization Moderate levels of testosterone-derived estradiol during the critical period are sufficient for defeminization of the brain. Defeminization of the rodent brain results in loss of estrogen positive feedback on LH and FSH secretion and the ensuing loss of cyclicity, as well as loss of female sex behavior. Masculinization High levels of estradiol due to high levels of testosterone during the critical period results in masculinization of the brain. Masculinization leads to the induction of male sex behavior including antagonism towards other males and the mounting of females.
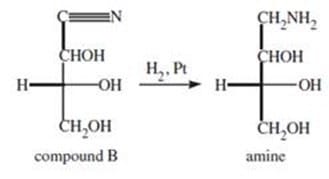
The conversion of testosterone to estradiol is what type of reaction?
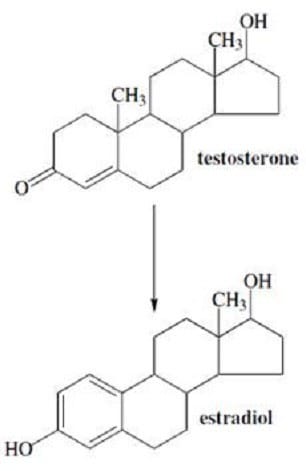
A. Reduction
B. Aromatization
C. Electrophilic aromatic substitution
D. Methylation
-
Question 485:
Sugars are carbohydrates, that is, molecules usually with the empirical formula C(H2O), and structural formulas made up of polyhydroxy aldehydes or ketones. Because of their polyfunctional nature, sugars can undergo a wide variety of
transformations upon treatment with acids, bases, or heat, and upon reaction with other simple reagents and enzymes. While many sugars occur in nature and are thus readily available, the synthesis and modification of simple sugars is a
necessary step in studies of enzymatic processes.
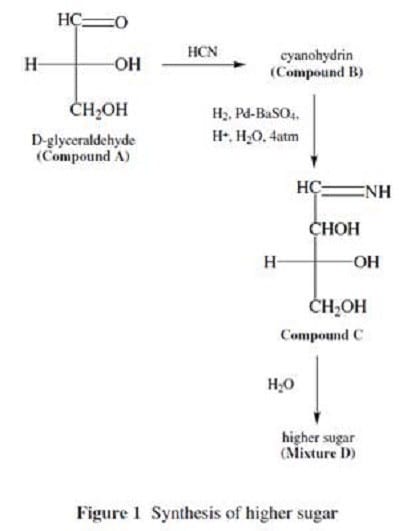
Higher sugars can be synthesized from the simple carbohydrate D-glyceraldehyde with the following procedure:
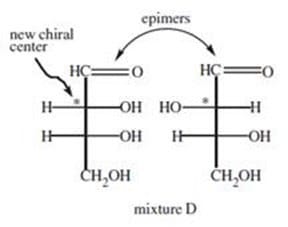
D-glyceraldehyde (Compound A) is reacted with HCN to produce a cyanohydrin (Compound B). Compound B is then treated with hydrogen gas and a modified palladium catalyst (similar to the Lindlar reagent) to give Compound C.
Compound C is hydrolyzed to give the higher sugars in Mixture D. This reaction is summarized in Figure 1. Mixture D contains two compounds, which can be separated by crystallization. Two doublets near 9.5 (, ppm) are observed in the 1H
NMR spectrum of mixture D, with each doublet corresponding to one of the two products present in the mixture. IR spectroscopy shows broad absorptions for both products around 3300 cm?.
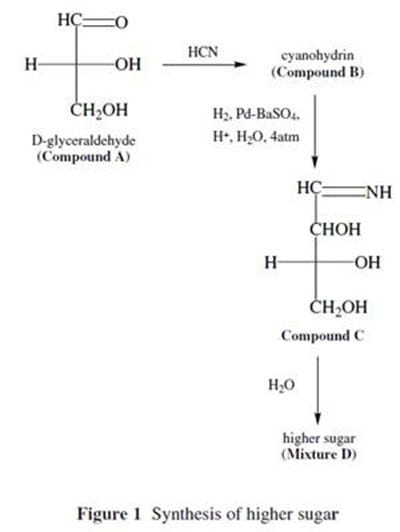
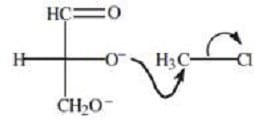
The hydroxyl groups of carbohydrates can also participate in reactions. For example, D-glyceraldehyde can react with chloromethane under basic conditions to yield a completely methylated product. This SN2 reaction is shown in Figure 2.
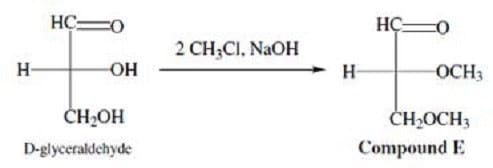
Figure 2 Methylation of D-glyceraldehyde
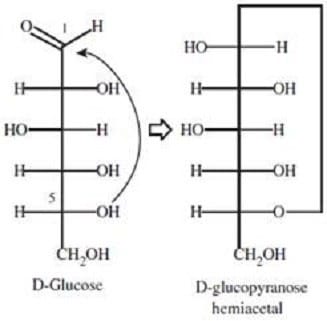
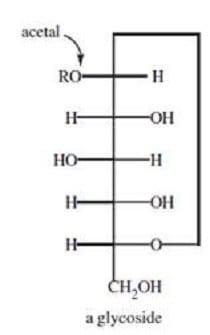
In glucose, the carbonyl carbon can be attacked, intramolecularly, by the hydroxyl oxygen of carbon-5 to form:
A. glucofuranose.
B. a hemiacetal.
C. a lactone.
D. a glycoside.
-
Question 486:
Sugars are carbohydrates, that is, molecules usually with the empirical formula C(H2O), and structural formulas made up of polyhydroxy aldehydes or ketones. Because of their polyfunctional nature, sugars can undergo a wide variety of
transformations upon treatment with acids, bases, or heat, and upon reaction with other simple reagents and enzymes. While many sugars occur in nature and are thus readily available, the synthesis and modification of simple sugars is a
necessary step in studies of enzymatic processes.
Higher sugars can be synthesized from the simple carbohydrate D-glyceraldehyde with the following procedure:
D-glyceraldehyde (Compound A) is reacted with HCN to produce a cyanohydrin (Compound B). Compound B is then treated with hydrogen gas and a modified palladium catalyst (similar to the Lindlar reagent) to give Compound C.
Compound C is hydrolyzed to give the higher sugars in Mixture D. This reaction is summarized in Figure 1. Mixture D contains two compounds, which can be separated by crystallization. Two doublets near 9.5 (, ppm) are observed in the 1H
NMR spectrum of mixture D, with each doublet corresponding to one of the two products present in the mixture. IR spectroscopy shows broad absorptions for both products around 3300 cm?.

The hydroxyl groups of carbohydrates can also participate in reactions. For example, D-glyceraldehyde can react with chloromethane under basic conditions to yield a completely methylated product. This SN2 reaction is shown in Figure 2.

Figure 2 Methylation of D-glyceraldehyde
What are the missing compounds, respectively, in the following reaction?
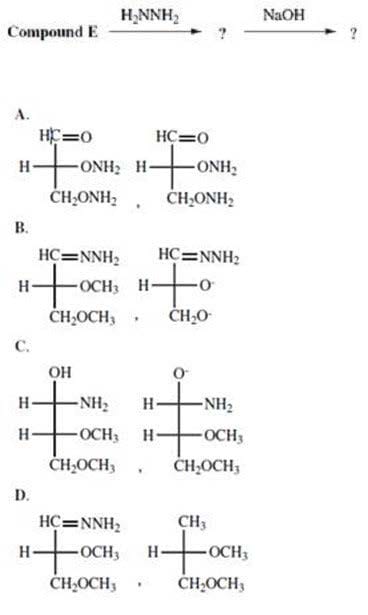
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 487:
Sugars are carbohydrates, that is, molecules usually with the empirical formula C(H2O), and structural formulas made up of polyhydroxy aldehydes or ketones. Because of their polyfunctional nature, sugars can undergo a wide variety of
transformations upon treatment with acids, bases, or heat, and upon reaction with other simple reagents and enzymes. While many sugars occur in nature and are thus readily available, the synthesis and modification of simple sugars is a
necessary step in studies of enzymatic processes.
Higher sugars can be synthesized from the simple carbohydrate D-glyceraldehyde with the following procedure:
D-glyceraldehyde (Compound A) is reacted with HCN to produce a cyanohydrin (Compound B). Compound B is then treated with hydrogen gas and a modified palladium catalyst (similar to the Lindlar reagent) to give Compound C.
Compound C is hydrolyzed to give the higher sugars in Mixture D. This reaction is summarized in Figure 1. Mixture D contains two compounds, which can be separated by crystallization. Two doublets near 9.5 (, ppm) are observed in the 1H
NMR spectrum of mixture D, with each doublet corresponding to one of the two products present in the mixture. IR spectroscopy shows broad absorptions for both products around 3300 cm?.
The hydroxyl groups of carbohydrates can also participate in reactions. For example, D-glyceraldehyde can react with chloromethane under basic conditions to yield a completely methylated product. This SN2 reaction is shown in Figure 2.

Figure 2 Methylation of D-glyceraldehyde The two sugars in Mixture D are:
A. enantiomers.
B. anomers.
C. epimers.
D. disaccharides.
-
Question 488:
Sugars are carbohydrates, that is, molecules usually with the empirical formula C(H2O), and structural formulas made up of polyhydroxy aldehydes or ketones. Because of their polyfunctional nature, sugars can undergo a wide variety of
transformations upon treatment with acids, bases, or heat, and upon reaction with other simple reagents and enzymes. While many sugars occur in nature and are thus readily available, the synthesis and modification of simple sugars is a
necessary step in studies of enzymatic processes.
Higher sugars can be synthesized from the simple carbohydrate D-glyceraldehyde with the following procedure:
D-glyceraldehyde (Compound A) is reacted with HCN to produce a cyanohydrin (Compound B). Compound B is then treated with hydrogen gas and a modified palladium catalyst (similar to the Lindlar reagent) to give Compound C.
Compound C is hydrolyzed to give the higher sugars in Mixture D. This reaction is summarized in Figure 1. Mixture D contains two compounds, which can be separated by crystallization. Two doublets near 9.5 (, ppm) are observed in the 1H
NMR spectrum of mixture D, with each doublet corresponding to one of the two products present in the mixture. IR spectroscopy shows broad absorptions for both products around 3300 cm?.

The hydroxyl groups of carbohydrates can also participate in reactions. For example, D-glyceraldehyde can react with chloromethane under basic conditions to yield a completely methylated product. This SN2 reaction is shown in Figure 2.
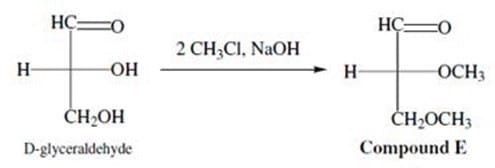
Figure 2 Methylation of D-glyceraldehyde
The reaction shown in Figure 2 most likely proceeds via the formation of a(n):
A. water leaving group.
B. nucleophilic alkoxide ion.
C. carbocation intermediate.
D. tetrahedral intermediate.
-
Question 489:
Sugars are carbohydrates, that is, molecules usually with the empirical formula C(H2O), and structural formulas made up of polyhydroxy aldehydes or ketones. Because of their polyfunctional nature, sugars can undergo a wide variety of
transformations upon treatment with acids, bases, or heat, and upon reaction with other simple reagents and enzymes. While many sugars occur in nature and are thus readily available, the synthesis and modification of simple sugars is a
necessary step in studies of enzymatic processes.
Higher sugars can be synthesized from the simple carbohydrate D-glyceraldehyde with the following procedure:
D-glyceraldehyde (Compound A) is reacted with HCN to produce a cyanohydrin (Compound B). Compound B is then treated with hydrogen gas and a modified palladium catalyst (similar to the Lindlar reagent) to give Compound C.
Compound C is hydrolyzed to give the higher sugars in Mixture D. This reaction is summarized in Figure 1. Mixture D contains two compounds, which can be separated by crystallization. Two doublets near 9.5 (, ppm) are observed in the 1H
NMR spectrum of mixture D, with each doublet corresponding to one of the two products present in the mixture. IR spectroscopy shows broad absorptions for both products around 3300 cm?.

The hydroxyl groups of carbohydrates can also participate in reactions. For example, D-glyceraldehyde can react with chloromethane under basic conditions to yield a completely methylated product. This SN2 reaction is shown in Figure 2.

Figure 2 Methylation of D-glyceraldehyde
The modified palladium catalyst is used instead of platinum to:
A. avoid reduction of the cyanohydrin to the amine.
B. make the reaction energetically favorable.
C. act as a Lewis acid.
D. reduce the likelihood of attack at the carbonyl carbon.
-
Question 490:
Which of the following reactants should be used to form the product shown below?
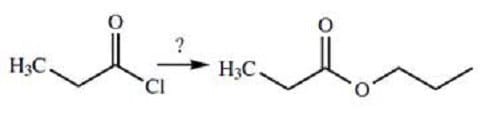
A. Sodium ethanoate
B. 1-propanol
C. Ammonia and ethane
D. Dimethyl ether
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.