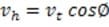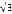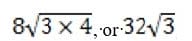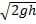Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 331:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30° to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45° to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.

Figure 1
The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp,
thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
How would the work done by gravity on the skier when she skis down the track compare with the work done by gravity on the skier if she fell the same vertical height?
A. Less work would be done on the skier when she skis down the track.
B. More work would be done on the skier when she skis down the track.
C. Equal amounts of work would be done.
D. The answer depends on the angle of the track.
-
Question 332:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30° to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45° to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.

Figure 1
The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp,
thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
Another ski jumper sets off from a point farther down the jump track, and leaves the ramp at a speed of 16 m/s. If the time in flight is 4 s, what is the total horizontal distance traveled by the ski jumper after leaving the ramp?
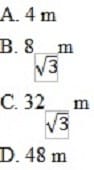
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 333:
Aski jump is an inclined track from which a ski jumper takes off through the air. After traveling down the track, the skier takes off from a ramp at the bottom of the track. The skier lands farther down on the slope.
Figure 1 shows a ski jump, in which the ramp at the lower end of the track makes an angle of 30?to the horizontal. The track is inclined at an angle of to the horizontal and the slope is inclined at an angle of 45?to the horizontal. A ski jumper is stationary at the top of the track. Once the skier pushes off, she accelerates down the track, and then takes off from the ramp. The vertical height difference between the top of the track and its lowest point is 50 m, and the vertical height difference between the top of the ramp and its lowest point is 10 m.

Figure 1
The distance traveled by the skier between leaving the ski jump ramp and making contact with the slope is called the jump distance. In some cases, in order to increase the jump distance a skier will jump slightly upon leaving the ramp,
thereby increasing the vertical velocity. Unless otherwise stated, assume that friction between the skis and the slope is negligible, and ignore the effects of air resistance.
How would the speed of a skier leaving the jump ramp change if the vertical height of the jump ramp were increased from its original height of 10 meters?
A. increase
B. decrease
C. remain the same
D. The answer depends on the incline angle of the jump ramp.
-
Question 334:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: or bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their component atomic orbitals, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz orbitals (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows:
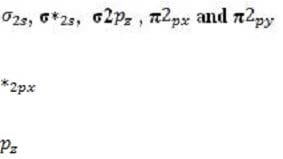
(equal in energy), and * (equal in energy), *2 . (The designation of the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
The quantum number that distinguishes the px orbital from the py orbital is called the:
A. azimuthal quantum number.
B. magnetic quantum number.
C. principal quantum number.
D. spin quantum number.
-
Question 335:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: or bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their component atomic orbitals, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz orbitals (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows:
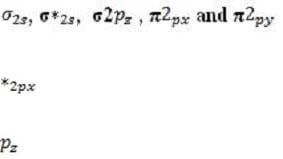
(equal in energy), and * (equal in energy), *2 . (The designation of the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
Molecular orbitals in hydrocarbons are formed between the 1s atomic orbital of hydrogen and the sp, sp2, or sp3 hybrid atomic orbitals of carbon. Which choice correctly lists the energy level of the C-H bonds, from lowest to highest?
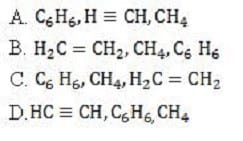
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 336:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: or bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their component atomic orbitals, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz orbitals (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows:
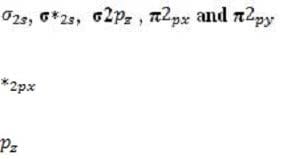
(equal in energy), and * (equal in energy), *2 . (The designation of the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
Which of the following figures describes the shape of *2pz molecular orbital?
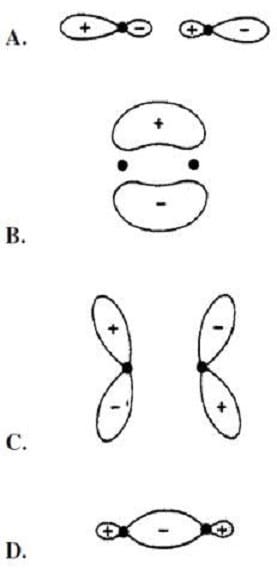
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 337:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: or bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their component atomic orbitals, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz orbitals (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows:
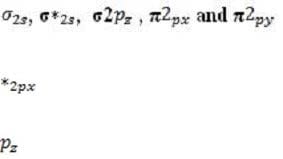
(equal in energy), and * (equal in energy), *2 . (The designation of the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
Given the order in which orbitals are filled, which molecule is a triplet in its ground state?
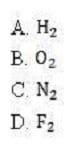
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 338:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: or bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their component atomic orbitals, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz orbitals (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows:
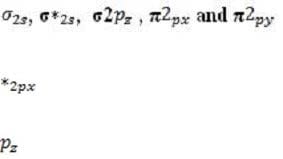
(equal in energy), and * (equal in energy), *2 . (The designation of the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
Among conjugated polyenes (molecules with alternating carbon-carbon double and single bonds) why are those that are longer able to absorb longer wavelengths of light?
A. Larger molecular orbitals have a lower ground state.
B. A longer wavelength is better able to interact with a longer molecular orbital.
C. The larger number of molecular orbitals allows for smaller energy transitions.
D. Larger molecular orbitals can absorb more energy.
-
Question 339:
Every atomic orbital contains plus and minus regions, defined by the value of the quantum mechanical function for electron density. When orbitals from different atoms overlap to form bonds, an equal number of new molecular orbitals results. These are of two types: bonding orbitals, formed by overlap between orbital regions with the same sign, and antibonding * or * orbitals, formed by overlap between regions with opposite signs. Bonding orbitals have lower energy than their
component atomic orbitals
, and antibonding orbitals have higher energy. The electron pairs reside in the lower-energy bonding orbitals; the higher-energy, less stable orbitals remain empty when the molecule is in its ground state. A benzene ring has six unhybridized pz (one from each carbon atom), which together from six molecular orbitals, each one delocalized over the entire ring. Of the possible orbital structures for benzene, the one with the lowest energy has the plus region of all six p orbital functions on one side of the ring. The six electrons occupying the orbitals fill the three most stable molecular orbitals, leaving the other three empty. Molecular orbitals are filled from the lowest to the highest energy level. The number of bonds between atoms is determined by the number of filled bonding orbitals minus the number of filled antibonding orbitals; each antibonding orbital

cancels out a filled bonding orbital. For a diatomic molecule, orbitals in the n = 2 energy level are filled as follows: , , 2 , and (equal in energy), and * (equal in energy), *2 . (The designation of
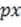
the three p orbitals as , , and are interchangeable.) Absorption of a photon can raise an electron to a higher-energy molecular orbital. The excited electron does not immediately change its spin, which is opposite to that of the electron with which it was previously paired. This singlet state is relatively unstable: the molecule may interact with another molecule, or fluoresce and return to its ground state. Alternatively, there may be a change in spin direction somewhere in the system; the molecule then enters the so-called triplet state, which generally has lower energy. The molecule now cannot return quickly to its ground state, since the excited electron no longer has a partner of opposite spin with which to pair. It also cannot return to the singlet state, because the singlet has greater energy. Consequently, the triplet state, which has two unpaired electrons in separate orbitals, is long-lived by atomic standards, with a lifetime that may be ten seconds or more. During this period, the molecule is highly reactive.
Which of the following four depictions of molecular orbitals represents the highest energy state for a 6- carbon polyene molecule? (The signs given are the signs for the mathematical functions defining the p orbitals on one side of the molecule.)
A. – – – – – –
B. + + + – – –
C. + + – – + +
D. + – + – + –
-
Question 340:
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.
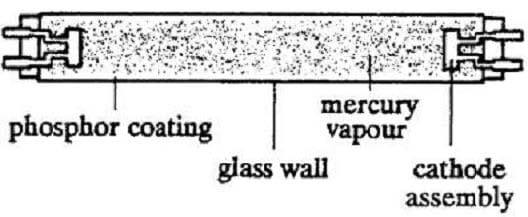
The lamp also emits a small proportion of ultraviolet light in addition to the light emitted in the visible spectrum. This ultraviolet light is incident on a metal that has a work function, which is the minimum energy necessary to free an electron, of
2.00 eV. What will be the kinetic energy of an electron that is ejected from the metal if the frequency of the incident light is 1.2?015 Hz? (Note: h = 4.14?0-15 eV穝.)?
A. 9.936 eV
B. 6.948 eV
C. 4.968 eV
D. 2.968 eV
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.