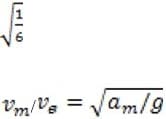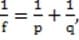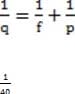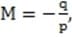Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 341:
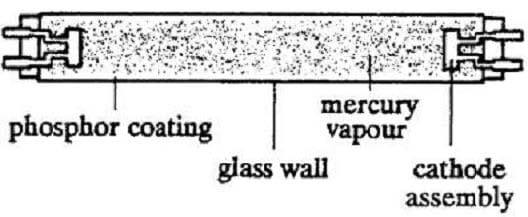
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.
In the phosphor coating, an electron falls from an excited state to a lower energy state, emitting a photon with an energy of 2.07 eV. What is the wavelength of the light emitted by the fluorescent tube? (Note: Planck's constant h = 4.14?0-15 eV穝, and c = 3?08m/s.)
A. 300 nm
B. 600 nm
C. 900 nm
D. 1242 nm
-
Question 342:
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.

Some fluorescent light bulbs are observed to glow for a short period after their power supply has been turned off. This glow is generated mainly by:
A. the incandescence of the hot ionic gas within the bulb surface.
B. emission of light stored as vibrational kinetic energy in the phosphor coating.
C. the dissipation of electric charge built up on the bulb's surface.
D. electrons returning to the ground state from excited states after the power was shut off.
-
Question 343:
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.

As the excited electrons in the coating drop back to their ground states in more than one step, they will emit light of:
A. higher frequency than the light absorbed.
B. longer wavelength than the light absorbed.
C. the same wavelength as the light absorbed.
D. greater energy than the light absorbed.
-
Question 344:
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.

If the fluorescent light is left on for 4 hours, how much useful energy is emitted as light?
A. 144 kJ
B. 432 kJ
C. 576 kJ
D. 900 kJ
-
Question 345:
Many nutrients required by plants exist in soil as basic cations:
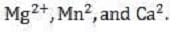
A soil's cation-exchange capacity is a measure of its ability to adsorb these basic cations as well as exchangeable hydrogen and aluminum ions. The cation-exchange capacity of soil is derived from two sources: small clay particles called micelles consisting of alternating layers of alumina and silica crystals, and organic colloids.
Replacement of + and + by other cations of lower valence creates a net negative charge within the inner layers of the micelles. This is called the soil's permanent charge. For example, replacement of an atom of aluminum by calcium within a section where the net charge was previously zero, as shown below, produces a net charge of ?, to which other cations can become adsorbed.
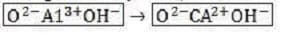
Figure 1
A pH-dependent charge develops when hydrogen dissociates from hydroxyl moieties on the outer surfaces of the clay micelles. This leaves negatively-charged oxygen atoms to which basic cations may adsorb. Likewise, a large pH-
dependent charge develops when hydrogen dissociates from carboxylic acids and phenols in organic matter.
In most clays, permanent charges brought about by substitution account for anywhere from half to nearly all of the total cation-exchange capacity. Soils very high in organic matter contain primarily pH-dependent charges. In a research study,
three samples of soil were leached with a 1 N solution of neutral KCl, and the displaced A13+ and basic cations measured. The sample was then leached again with a buffered solution of BaCl2 and triethanolamine at pH 8.2, and the
displaced H+ measured. Table 1 gives results for three soils tested by this method.
Table 1
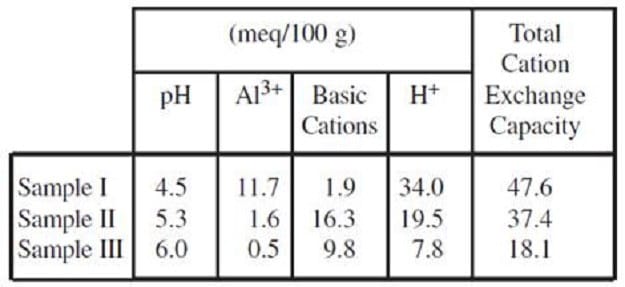
Due to the buffering effect of the soil's cation exchange capacity, just measuring the soil solution's pH will not indicate how much base is needed to change the soil pH. In another experiment, measured amounts of acid and base were added to 10-gram samples of well-mixed soil that had been collected from various locations in a field. The volumes of the samples were equalized by adding water. The results were recorded in Figure 2.
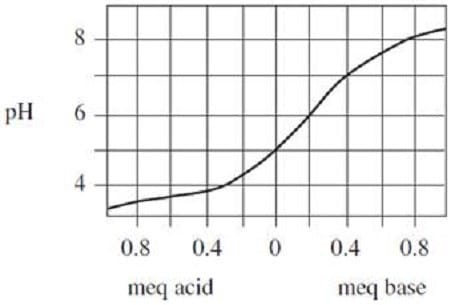
Figure 2.
A body is dropped from a height of 30 m on Earth and hits the ground with a velocity. The body is then taken to the Moon, which has a gravitational acceleration 1/6 that of Earth. It is again dropped from a height of 30 m, hitting the Moon with a velocity of vm. What is the ratio of vm/ve?
A. 1/6
B. sqrt (1/6)
C. 6
D. 36
-
Question 346:
When light in the ultraviolet region of the spectrum is shone on a type of material known as a phosphor, it fluoresces and emits light in the visible region of the spectrum. Lamps that utilize this property, known as fluorescent lamps, are very efficient light sources. The arrangement of a typical fluorescent lamp is shown below. The lamp is a glass tube whose inside walls are covered with a phosphor. The tube has an appreciable length-to-diameter ratio so as to reduce the power losses at each end, and it is filled with argon gas mixed with mercury vapor. Inside each end of the tube are tungsten electrodes covered with an emission material.
Electrons are liberated at the cathode and accelerated by an applied electric field. These free electrons encounter the gas mixture, ionizing some mercury atoms and exciting others. Since it requires more energy to ionize the atoms than to excite the electrons, more excitation than ionization occurs. When the excited electrons revert to their ground state, they radiate ultraviolet photons with a wavelength of 253.7 nm. These photons impinge on the phosphor coating of the tube and excite electrons in the phosphor to higher energy states. The excited electrons in the phosphor return to their ground state in two or more steps, producing radiation in the visible region of the spectrum. Not every fluorescent lamp emits the same color of radiation; the color is dependent on the relative percentages of different heavy metal compounds in the phosphor.
The fluorescent lamp shown operates at 100 volts and draws 400 milliamps of current during normal operation. Of the total power that the lamp consumes, only 25% is converted to light, while the remaining 75% is dissipated as heat. This energy keeps the lamp at its optimum working temperature of 40°C. In the lamp shown, the phosphor coating is calcium metasilicate, which emits orange to yellow light.

The photons emitted by the mercury vapor have energies:
A. equal to the energies of the electric current.
B. equal to the voltage across the tube.
C. equal to the energy differences between electron orbitals in the mercury atom.
D. less than or equal to the energy differences between the electron orbitals of the mercury atom.
-
Question 347:
Many nutrients required by plants exist in soil as basic cations:
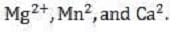
A soil's cation-exchange capacity is a measure of its ability to adsorb these basic cations as well as exchangeable hydrogen and aluminum ions. The cation-exchange capacity of soil is derived from two sources: small clay particles called micelles consisting of alternating layers of alumina and silica crystals, and organic colloids.
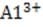
Replacement of + and + by other cations of lower valence creates a net negative charge within the inner layers of the micelles. This is called the soil's permanent charge. For example, replacement of an atom of aluminum by calcium within a section where the net charge was previously zero, as shown below, produces a net charge of ?, to which other cations can become adsorbed.
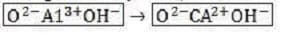
Figure 1
A pH-dependent charge develops when hydrogen dissociates from hydroxyl moieties on the outer surfaces of the clay micelles. This leaves negatively-charged oxygen atoms to which basic cations may adsorb. Likewise, a large pH-
dependent charge develops when hydrogen dissociates from carboxylic acids and phenols in organic matter.
In most clays, permanent charges brought about by substitution account for anywhere from half to nearly all of the total cation-exchange capacity. Soils very high in organic matter contain primarily pH-dependent charges. In a research study,
three samples of soil were leached with a 1 N solution of neutral KCl, and the displaced A13+ and basic cations measured. The sample was then leached again with a buffered solution of BaCl2 and triethanolamine at pH 8.2, and the
displaced H+ measured. Table 1 gives results for three soils tested by this method.
Table 1

Due to the buffering effect of the soil's cationexchange capacity, just measuring the soil solution's pH will not indicate how much base is needed to change the soil pH. In another experiment, measured amounts of acid and base were added to 10-gram samples of well-mixed soil that had been collected from various locations in a field. The volumes of the samples were equalized by adding water. The results were recorded in Figure 2.

Figure 2.
An electron travels in the plane of the page from left to right, perpendicular to a magnetic field that points into the page. The direction of the resulting magnetic force on the electron will be in the plane of the page and:
A. upwards.
B. downwards.
C. to the left.
D. to the right.
-
Question 348:
Many nutrients required by plants exist in soil as basic cations:
A soil's cation-exchange capacity is a measure of its ability to adsorb these basic cations as well as exchangeable hydrogen and aluminum ions. The cation-exchange capacity of soil is derived from two sources: small clay particles called micelles consisting of alternating layers of alumina and silica crystals, and organic colloids.
Replacement of + and + by other cations of lower valence creates a net negative charge within the inner layers of the micelles. This is called the soil's permanent charge. For example, replacement of an atom of aluminum by calcium within a section where the net charge was previously zero, as shown below, produces a net charge of ?, to which other cations can become adsorbed.
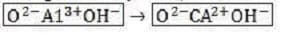
Figure 1
A pH-dependent charge develops when hydrogen dissociates from hydroxyl moieties on the outer surfaces of the clay micelles. This leaves negatively-charged oxygen atoms to which basic cations may adsorb. Likewise, a large pH-
dependent charge develops when hydrogen dissociates from carboxylic acids and phenols in organic matter.
In most clays, permanent charges brought about by substitution account for anywhere from half to nearly all of the total cation-exchange capacity. Soils very high in organic matter contain primarily pH-dependent charges. In a research study,
three samples of soil were leached with a 1 N solution of neutral KCl, and the displaced A13+ and basic cations measured. The sample was then leached again with a buffered solution of BaCl2 and triethanolamine at pH 8.2, and the
displaced H+ measured. Table 1 gives results for three soils tested by this method.
Table 1

Due to the buffering effect of the soil's cation exchange capacity, just measuring the soil solution's pH will not indicate how much base is needed to change the soil pH. In another experiment, measured amounts of acid and base were added to 10-gram samples of well-mixed soil that had been collected from various locations in a field. The volumes of the samples were equalized by adding water. The results were recorded in Figure 2.

Figure 2.
How much solid NaOH is required to neutralize 700 mL of 2 N HNO3?
A. 40 g
B. 48 g
C. 56 g
D. 64 g
-
Question 349:
Many nutrients required by plants exist in soil as basic cations:
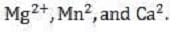
A soil's cation-exchange capacity is a measure of its ability to adsorb these basic cations as well as exchangeable hydrogen and aluminum ions. The cation-exchange capacity of soil is derived from two sources: small clay particles called micelles consisting of alternating layers of alumina and silica crystals, and organic colloids.
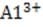
Replacement of + and + by other cations of lower valence creates a net negative charge within the inner layers of the micelles. This is called the soil's permanent charge. For example, replacement of an atom of aluminum by calcium within a section where the net charge was previously zero, as shown below, produces a net charge of ?, to which other cations can become adsorbed.
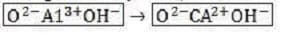
Figure 1
A pH-dependent charge develops when hydrogen dissociates from hydroxyl moieties on the outer surfaces of the clay micelles. This leaves negatively-charged oxygen atoms to which basic cations may adsorb. Likewise, a large pH-
dependent charge develops when hydrogen dissociates from carboxylic acids and phenols in organic matter.
In most clays, permanent charges brought about by substitution account for anywhere from half to nearly all of the total cation-exchange capacity. Soils very high in organic matter contain primarily pH-dependent charges. In a research study,
three samples of soil were leached with a 1 N solution of neutral KCl, and the displaced A13+ and basic cations measured. The sample was then leached again with a buffered solution of BaCl2 and triethanolamine at pH 8.2, and the
displaced H+ measured. Table 1 gives results for three soils tested by this method.
Table 1

Due to the buffering effect of the soil's cation exchange capacity, just measuring the soil solution's pH will not indicate how much base is needed to change the soil pH. In another experiment, measured amounts of acid and base were added to 10-gram samples of well-mixed soil that had been collected from various locations in a field. The volumes of the samples were equalized by adding water. The results were recorded in Figure 2.
Figure 2.

If 29 g of maleic acid () is dissolved in 500 g of ammonia (N), what is the molality of the resulting solution?
A. 0.05 m
B. 0.10 m
C. 0.25 m
D. 0.50 m
-
Question 350:
Many nutrients required by plants exist in soil as basic cations:
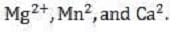
A soil's cation-exchange capacity is a measure of its ability to adsorb these basic cations as well as exchangeable hydrogen and aluminum ions. The cation-exchange capacity of soil is derived from two sources: small clay particles called micelles consisting of alternating layers of alumina and silica crystals, and organic colloids.
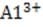
Replacement of + and + by other cations of lower valence creates a net negative charge within the inner layers of the micelles. This is called the soil's permanent charge. For example, replacement of an atom of aluminum by calcium within a section where the net charge was previously zero, as shown below, produces a net charge of ?, to which other cations can become adsorbed.
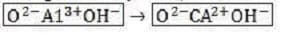
Figure 1
A pH-dependent charge develops when hydrogen dissociates from hydroxyl moieties on the outer surfaces of the clay micelles. This leaves negatively-charged oxygen atoms to which basic cations may adsorb. Likewise, a large pH-
dependent charge develops when hydrogen dissociates from carboxylic acids and phenols in organic matter.
In most clays, permanent charges brought about by substitution account for anywhere from half to nearly all of the total cation-exchange capacity. Soils very high in organic matter contain primarily pH-dependent charges. In a research study,
three samples of soil were leached with a 1 N solution of neutral KCl, and the displaced A13+ and basic cations measured. The sample was then leached again with a buffered solution of BaCl2 and triethanolamine at pH 8.2, and the
displaced H+ measured. Table 1 gives results for three soils tested by this method.
Table 1

Due to the buffering effect of the soil's cation exchange capacity, just measuring the soil solution's pH will not indicate how much base is needed to change the soil pH. In another experiment, measured amounts of acid and base were added to 10-gram samples of well-mixed soil that had been collected from various locations in a field. The volumes of the samples were equalized by adding water. The results were recorded in Figure 2.
Figure 2.

A converging lens has a focal length of 8 cm. If the object is 10 cm to the left of the lens, what are the position of the image formed and the magnification of the lens?
A. 0.025 cm to the right of the lens and 0.0025X
B. 4.4 cm to the right of the lens and 0.4X
C. 40 cm to the right of the lens and 4X
D. 40 cm to the left of the lens and 4X
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.