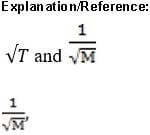Exam Details
Exam Code
:MCAT-TESTExam Name
:Medical College Admission Test: Verbal Reasoning, Biological Sciences, Physical Sciences, Writing SampleCertification
:Medical Tests CertificationsVendor
:Medical TestsTotal Questions
:812 Q&AsLast Updated
:Apr 16, 2025
Medical Tests Medical Tests Certifications MCAT-TEST Questions & Answers
-
Question 361:
The periodic beating of the heart is controlled by electrical impulses that originate within the cardiac muscle itself. These pulses travel to the sinoatrial node and from there to the atria and the ventricles, causing the cardiac muscles to contract. If a current of a few hundred milliamperes passes through the heart, it will interfere with this natural system, and may cause the heart to beat erratically. This condition is known as ventricular fibrillation, and is life-threatening. If, however, a larger current of about 5 to 6 amps is passed through the heart, a sustained ventricular contraction will occur. The cardiac muscle cannot relax, and the heart stops beating. If at this point the muscle is allowed to relax, a regular heartbeat will usually resume.
The large current required to stop the heart is supplied by a device known as a defibrillator. A schematic diagram of a defibrillator is shown below. This device is essentially a "heavy-duty" capacitor capable of storing large amounts of energy. To charge the capacitor quickly (in 1 to 3 seconds), a large DC voltage must be applied to the plates of the capacitor. This is achieved using a step-up transformer, which creates an output voltage that is much larger than the input voltage. The transformer used in this defibrillator has a step-up ratio of 1:50.
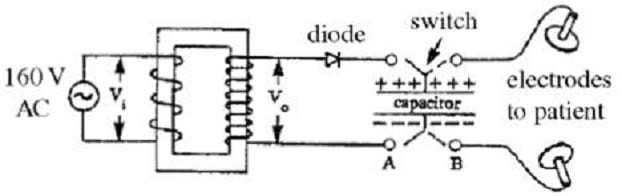
The AC voltage that is obtained from the transformer must then be converted to DC voltage in order to charge the capacitor. This is accomplished using a diode, which allows current flow in one direction only. Once the capacitor is fully charged, the charge remains stored until the switch is moved to position B and the plates are placed on the patient's chest. To cut down the resistance between the patient's body and the defibrillator, the electrodes are covered with a wetting gel before use. Care must be taken to insure that the patient is not in electrical contact with the ground while the defibrillator is in use.
Why is it important to insure that the patient is not in electrical contact with the ground while the defibrillator is in use?
A. Contact with the ground will decrease the resistance across the patient's body.
B. The doctor administering the treatment will be in greater danger of receiving an electric shock if the patient is in electrical contact with the ground.
C. Contact with the ground will cause a smaller current to pass through the patient's heart.
D. The patient receiving the treatment will be in greater danger of receiving burns due to the high current density if he is in electrical contact with the ground.
-
Question 362:
The periodic beating of the heart is controlled by electrical impulses that originate within the cardiac muscle itself. These pulses travel to the sinoatrial node and from there to the atria and the ventricles, causing the cardiac muscles to contract. If a current of a few hundred milliamperes passes through the heart, it will interfere with this natural system, and may cause the heart to beat erratically. This condition is known as ventricular fibrillation, and is life-threatening. If, however, a larger current of about 5 to 6 amps is passed through the heart, a sustained ventricular contraction will occur. The cardiac muscle cannot relax, and the heart stops beating. If at this point the muscle is allowed to relax, a regular heartbeat will usually resume.
The large current required to stop the heart is supplied by a device known as a defibrillator. A schematic diagram of a defibrillator is shown below. This device is essentially a "heavy-duty" capacitor capable of storing large amounts of energy. To charge the capacitor quickly (in 1 to 3 seconds), a large DC voltage must be applied to the plates of the capacitor. This is achieved using a step-up transformer, which creates an output voltage that is much larger than the input voltage. The transformer used in this defibrillator has a step-up ratio of 1:50.

The AC voltage that is obtained from the transformer must then be converted to DC voltage in order to charge the capacitor. This is accomplished using a diode, which allows current flow in one direction only. Once the capacitor is fully charged, the charge remains stored until the switch is moved to position B and the plates are placed on the patient's chest. To cut down the resistance between the patient's body and the defibrillator, the electrodes are covered with a wetting gel before use. Care must be taken to insure that the patient is not in electrical contact with the ground while the defibrillator is in use.
If the defibrillator has a capacitance of 10 F, how much charge will build up on the two plates?
A. 0.08 coulombs
B. 0.8 coulombs
-
Question 363:
The periodic beating of the heart is controlled by electrical impulses that originate within the cardiac muscle itself. These pulses travel to the sinoatrial node and from there to the atria and the ventricles, causing the cardiac muscles to contract. If a current of a few hundred milliamperes passes through the heart, it will interfere with this natural system, and may cause the heart to beat erratically. This condition is known as ventricular fibrillation, and is life-threatening. If, however, a larger current of about 5 to 6 amps is passed through the heart, a sustained ventricular contraction will occur. The cardiac muscle cannot relax, and the heart stops beating. If at this point the muscle is allowed to relax, a regular heartbeat will usually resume.
The large current required to stop the heart is supplied by a device known as a defibrillator. A schematic diagram of a defibrillator is shown below. This device is essentially a "heavy-duty" capacitor capable of storing large amounts of energy. To charge the capacitor quickly (in 1 to 3 seconds), a large DC voltage must be applied to the plates of the capacitor. This is achieved using a step-up transformer, which creates an output voltage that is much larger than the input voltage. The transformer used in this defibrillator has a step-up ratio of 1:50.

The AC voltage that is obtained from the transformer must then be converted to DC voltage in order to charge the capacitor. This is accomplished using a diode, which allows current flow in one direction only. Once the capacitor is fully charged, the charge remains stored until the switch is moved to position B and the plates are placed on the patient's chest. To cut down the resistance between the patient's body and the defibrillator, the electrodes are covered with a wetting gel before use. Care must be taken to insure that the patient is not in electrical contact with the ground while the defibrillator is in use.
The resistance between the two electrodes when placed apart on the patient's chest is 1,000 when wetting gel is used. What is the initial current through the patient's heart, assuming that all the current takes this path?
A. 0.16 A
B. 4 A
C. 6.25 A
D. 8 A
-
Question 364:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.
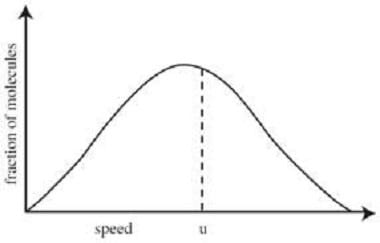
If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision. The average kinetic energy (E) of a gas particle is given by:
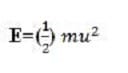
Equation 1
where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles:

where N is the number of gas particles; this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:
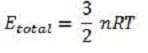
Equation 2
where n is the number of moles of gas. Combining these equations gives:
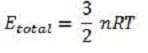
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
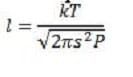
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
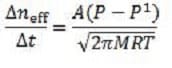
Equation 5
Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1.
Which of the following will have the smallest root mean square speed at 298K?
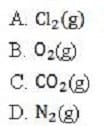
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 365:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.

If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision.
The average kinetic energy (E) of a gas particle is given by:
Equation 1
where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles:

where N is the number of gas particles; this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:
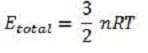
Equation 2
where n is the number of moles of gas. Combining these equations gives:
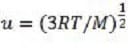
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
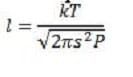
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
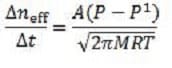
Equation 5
Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1.
What is the relative rate of effusion for a mixture of two noble gases, GA and GB, which escape through the same pinhole?
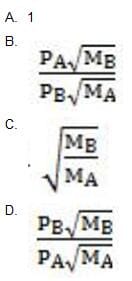
A. Option A
B. Option B
C. Option C
D. Option D
-
Question 366:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.

If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision. The average kinetic energy (E) of a gas particle is given by:
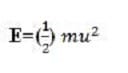
Equation 1
where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles:

where N is the number of gas particles; this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:
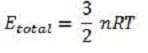
Equation 2
where n is the number of moles of gas. Combining these equations gives:
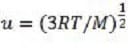
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
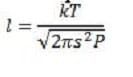
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
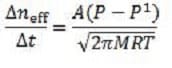
Equation 5
Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1.
The average kinetic energy of an ideal gas can be directly related to the:
A. rms speed.
B. temperature.
C. Boltzmann constant.
D. universal gas constant.
-
Question 367:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.

If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision. The average kinetic energy (E) of a gas particle is given by:
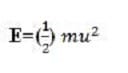
Equation 1
where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles: where N is the number of gas particles;

this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:
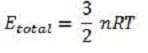
Equation 2
where n is the number of moles of gas. Combining these equations gives:
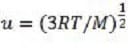
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
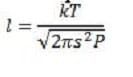
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
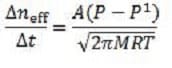
Equation 5
Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1.
The mean free path of a gas will be longer if the :
A. pressure of the gas is increased.
B. number of gas particles per unit volume is increased.
C. distance between collisions is decreased.
D. pressure of the gas is decreased.
-
Question 368:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.
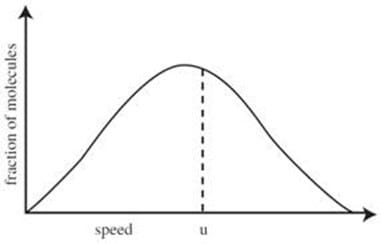
If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision. The average kinetic energy (E) of a gas particle is given by:
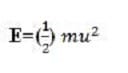
Equation 1
where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles:

where N is the number of gas particles; this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:
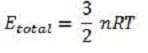
Equation 2
where n is the number of moles of gas. Combining these equations gives:
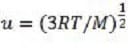
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
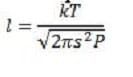
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
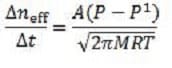
Equation 5
Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1.
Which of the following gives values for both standard temperature and pressure?
A. 273 K and 760 Torr
B. 273 K and 1 atm
C. 0°C and 760 mm Hg
D. All of the above
-
Question 369:
The equation of state of an ideal gas is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature of the gas. The gas particles in a container are constantly moving at various speeds. These speeds are
characterized by the Maxwell shown in the figure below.

If two particles collide, their velocities change. However, if the gas is in thermal equilibrium, the velocity distribution of the gas as a whole will remain unchanged by the collision. The average kinetic energy (E) of a gas particle is given by:
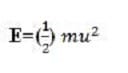
Equation 1 where m is the mass of one particle and u is the root mean square speed (rms speed) of the gas particles
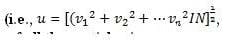
where N is the number of gas particles; this is different from the average speed). For an ideal gas, the kinetic energy of all the particles is:Z
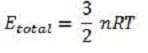
Equation 2
where n is the number of moles of gas. Combining these equations gives:
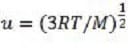
Equation 3
where M is the molar mass of the gas particles.
The average distance a particle travels between collisions is known as the mean free path l. Intuitively, the mean free path (mfp) could be expected to be larger for gases at low pressure, since there is a lot of space between particles.
Similarly, the mfp should be larger when the gas particles are small. The following expression for the mfp shows this to be correct.
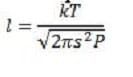
Equation 4 In this equation, s is the atomic diameter (typically on the order of 10?), k is the Boltzmann constant, and P is the pressure. In addition to colliding with one another, gas particles also collide with the walls of their container. If the container wall has a pinhole that is small compared to the mfp of the gas, and a pressure differential exists across the wall, the particles will effuse (or escape) through this pinhole without disturbing the Maxwellian distribution of the particles. The rate of effusion can be described by:
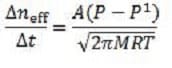
Equation 5 Where neff is the number of moles of effusing particles, A is the area of the pinhole, p and p1 are the pressures on the inside and outside of the container wall respectively, and p>p1. If a pinhole were made in a container containing a mixture of equal amounts of H2, O2, N2 and CO2, which gas would have the fastest effusion rate?
A. H2
B. O2
C. N2
D. Co2
-
Question 370:
Which of the following neurotransmitters could be imbalanced if someone woke up and realized they could not move any muscle?
A. Catecholamine
B. Acetylcholine
C. Frataxin
D. Cortisol
Related Exams:
Tips on How to Prepare for the Exams
Nowadays, the certification exams become more and more important and required by more and more enterprises when applying for a job. But how to prepare for the exam effectively? How to prepare for the exam in a short time with less efforts? How to get a ideal result and how to find the most reliable resources? Here on Vcedump.com, you will find all the answers. Vcedump.com provide not only Medical Tests exam questions, answers and explanations but also complete assistance on your exam preparation and certification application. If you are confused on your MCAT-TEST exam preparations and Medical Tests certification application, do not hesitate to visit our Vcedump.com to find your solutions here.